Electrostatic Generator
All material objects in our world are made up of atoms. Each atom, in turn, has in the middle a positively charged nucleus surrounded by negatively charged electrons. Atoms can become positively charged if they lose electrons or negatively charged if they capture extra electrons. These atoms are called ions. When something is made up of a number of ionized atoms, the thing itself has a charge. Because the charge itself is not moving, it is called a static charge (static means “not moving”) or “static electricity.” Negatively charge objects will attract positively charged objects. If they get too close the electrons will jump from the negative thing back to the positive thing in the form of a spark. This is a one-time deal; the electrons do not keep flowing, which they do in “current” electricity.
If you ever rubbed your feet on a rug and touched your friends, zapping them with sparks, then you have produced static electricity. Your feet produced frictional energy that stole the electrons away from the rug. If you rub a balloon against your hair, you will notice that the now positively charged balloon pulls your negatively charged hair toward it. You can also stick the balloon to the wall (most surfaces have a slight negative charge, because the electrons are on the outside of the atom).
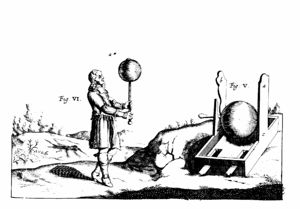
It was the ancient Greek scientists who first noticed that rubbing certain materials produced this effect, especially a material called amber, a fossilized resin that the Greeks used—and we still use—to make jewelry (in fact, “electron” means “amber” in Greek!). When people became interested in science again in 17th century Europe, they once again tried to study this effect, and wanted a way to produce it in a regular and sustained manner. In the mid 1660s, German Otto von Guericke (1602-1686) made a machine where a ball of sulfur was turned on an axle while a cloth rubbed on the ball’s surface. The ball became charged, gave off sparks, and attracted light pieces of straw. This was the first machine for producing static electricity, or the first electrostatic generator. Many developments of electrostatic generators followed. The invention in 1745 of the Leyden jar, an early capacitor, enabled the charges produced by the generators to be stored for large periods of time for experiments. Other improved static charge storage devices and electrostatic generators soon followed. A gift from an English friend to Benjamin Franklin of an electrostatic generator and a Leyden jar led to Franklin’s famous electrical experiments.
Even after Alessandro Volta made a source of current electricity available by inventing the battery (the Votaic pile) in 1799, scientists and engineers continued to experiment with static electricity and develop electrostatic generators. For example, in 1840 the English engineer William Armstrong (1810-1900) noticed steam leaking from a boiler. When he went to try to stop it, he got an electric shock. He realized that the steam was giving up electric charges as it expanded into the air. In 1842 he built a steam-powered electrostatic generator that was much more powerful than earlier models. However, by this time current electricity was already being used in the telegraph and in early experiments in arc lighting, so Armstrong’s machine remained a curiosity. A little later, though, physicists realized that large amounts of static electricity could help them in their efforts to study subatomic particles. In 1931, Robert J. Van de Graaff built a generator able to produce millions of volts of static electricity. It was really a fancy version of von Guerecke’s original machine—a motorized belt ran continuously over a metal brush. In physics, these Van de Graaff generators were later replaced by better machines, but if you go to your local science museum you will probably see a Van de Graaf generator demonstrating static electricity by making some kid’s hair stand on end.