Carbon Dioxide (CO₂) Capture and Storage (CCS)
The original version of this article was created by Francesco Gerali, 2020 Elizabeth & Emerson Pugh Scholar in Residence at the IEEE History Center
It is recommended this article be cited as:
F. Gerali (2019). Carbon Dioxide (CO2) Capture and Storage (CCS), Engineering and Technology History Wiki. [Online] Available: https://ethw.org/Carbon_Dioxide_(CO%E2%82%82)_Capture_and_Storage_(CCS)
Introduction
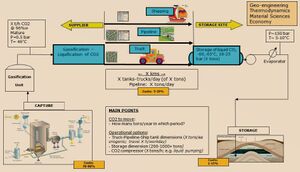
Carbon dioxide (CO2)[1] capture and storage (CCS) is a process consisting of I) the separation of CO2 from industrial and energy-related sources, II) transport to a subsoil storage location and III) long-term segregation from the atmosphere (Fig. 1). CO2 capture and storage has not to be confused with the expressions CO2 geological sequestration. This has almost fallen into disuse since the mid-2000s and is more relevant to a non-definitive carbon dioxide storage process.
Carbon dioxide derived from biological activity, igneous activity, and chemical reactions between rocks and fluids accumulates in the natural subsurface environment as carbonate minerals, in a solution or in a gaseous or supercritical form, either as a gaseous mixture or as pure CO2. The subsurface is the Earth’s largest carbon-dioxide reservoir. The vast majority of the world’s carbon is included in coals, petroleum, gas organic-rich shales and carbonate rocks. Geological storage of CO2 has been a natural process in the Earth’s upper crust for hundreds of millions of years.
Capture and geological storage of CO2 helps to mitigate the emissions of CO2 into the atmosphere, by capturing CO2 from major stationary sources, transporting it usually by pipeline and injecting it into suitable deep rock formations. The engineered injection of CO2 into subsurface geological formations was first undertaken in Texas, USA, in the early 1970s, as part of the enhanced oil recovery (EOR)[2] projects. Geological storage of human-produced CO2 as a greenhouse gas mitigation option was first proposed in the 1970s, but little research was done until the early 1990s, when the idea eventually gained credibility after 20 years of studies.
Since 1993, the main application of CO2 capture regards large point sources: fossil fuel power plants, fuel processing plants and other industrial plants, particularly for the manufacture of iron, steel, cements and chemicals. This is a brief chronology of the main forerunner projects on CO2 geological storage developed in Europe:
- British Geological Survey,[3] project Joule II, 1993-1995. Early feasibility study of underground CO2 storage. United Kingdom, CT92-0031.
- Statoil,[4] project SACS 1 Nov. 98 - Jan. 01. Goal: CO2 storage in a saline aquifer. Norway, OG/ 306/98 (demonstration held at the Sleipner camp).
- Statoil, project SACS 2 Apr. 00 - Mar. 02. Goal: CO2 storage in a saline aquifer Norway, ENK6-CT-1999-00014 (demonstration held at the Sleipner camp).
- GEUS,[5] project GESTCO Mar. 00 - Aug. 02. Goal: geological storage of CO2 from burning fossil fuels and other artificial sources. Denmark, ENK6-CT-1999-00010.
- Imperial College,[6] project ICBM Oct. 00 - Sept. 03. Goal: CO2 storage in deep coal fields, with further production of methane gas. UK.
- British Geological Survey, project NASCENT Jan. 01 Dec. - 03. Goal: study of natural CO2 deposits with and without associated surface manifestations of CO2. United Kingdom, NNE5-CT2000-00303.
- British Geological Survey, project WEYBURN Jan. 01 - June 04. Goal: CO2 storage in old oil fields, with further UK oil production, NNE5-CT2000-00304.
- British Petroleum,[7] project NGCAS, Oct. 2001 - Sept. 2004. Goal: risk analysis for the long- term geological storage of CO2. United Kingdom, NNE5/2000/90.
- TNO,[8] project RECOPOL, Nov. 2001 - Nov. 2004. Goal: CO2 storage in deep coal fields in Poland, producing methane. Holland, ENK- CT-2001-00539.
By the late 1990s, a number of government and privately funded research programs were under way in the United States, Canada, Japan, and Australia. Several oil & gas companies became increasingly interested in geological storage, particularly for gas fields with a high natural CO2 content such as Natuna in Indonesia, In Salah in Algeria and Gorgon in Australia. Research has progressed and as demonstration and commercial projects have been successfully undertaken, the level of confidence in the technology has increased.
CO2 Capture Systems
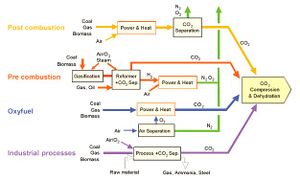
There are four basic systems for capturing CO2 from use of fossil fuels and biomass (Fig. 2):
- Capture from industrial process streams
- Post-combustion capture
- Oxy-fuel combustion capture
- Pre-combustion capture
1. Capture from industrial process streams
CO2 has been captured from industrial process streams for almost a century, but most was released into the atmosphere because there was no incentive or requirement to store it. Examples of CO2 capture from process streams are purification of natural gas and production of hydrogen-containing synthesis gas for the manufacture of ammonia, alcohols and synthetic liquid fuels, cement and steel production, and fermentation processes for food and drink production. CO2 could be captured from these streams using technologies that are common to post-combustion capture, oxyfuel combustion capture and pre-combustion capture systems.
2. Post-combustion capture
Capture of CO2 from flue gases[9] produced by combustion of fossil fuels and biomass in is called post-combustion capture. Instead of being discharged directly to the atmosphere, flue gases are passed through equipment which separates most of the CO2, which is then channeled to a storage reservoir and the remaining flue gas is discharged. A chemical sorbent technology would normally be used for CO2 separation.
3. Oxy-fuel combustion capture
In oxy-fuel combustion,[10] nearly pure oxygen is used for combustion instead of air, resulting in a CO2 and H2O flue gas. Oxygen is usually produced by cryogenic air separation. The power plant systems of reference for oxy-fuel combustion capture systems are the same utilized for post-combustion capture systems.
4. Pre-combustion capture
Pre-combustion capture implies the reaction of a fuel with oxygen or air and/or steam to obtain a “synthesis gas” (syngas)[11] composed of carbon monoxide and hydrogen. The carbon monoxide is mixed with steam in a catalytic reactor, called a shift converter.[12] CO2 is then separated, usually by a physical or chemical absorption process, resulting in a hydrogen-rich fuel that can be used in boilers, furnaces, gas turbines, engines and fuel cells.
CO2 Capture Technologies
CO2 capture systems have been put into practice since the early 1990s through the main known technologies for gas separation
- Separation with sorbents/solvents
- Separation with membranes
- Distillation of a liquefied gas stream and refrigerated separation
1. Separation with sorbents/solvents
Separation is obtained by passing the CO2-containing gas in contact with a liquid absorbent or solid sorbent[13] capable of gather the CO2. Other experimental processes based on new liquid sorbents, or new solid regenerable sorbents, were developed in early 2000s, and aimed to overcome the limitations of the existing systems. A problem with these CO2 capture technology emerged: the flow of the sorbent material between the vessels is large because it has to match with the high flow of CO2 in process. Consequently, equipment sizes and the energy required for sorbent regeneration are large and that results into low efficiency penalty and extra costs.
2. Separation with membranes
Membranes are specially manufactured materials that allow the selective permeation of a gas through them. The selectivity of the membrane is intimately related to the nature of the material, but the flow of gas through the membrane is usually driven by the pressure difference across the membrane. There are many different types of membrane materials (polymeric, metallic, or ceramic) that may find application in CO2 capture. Still at the end of 1990s membranes have not yet been applied for the large scale and demanding conditions in terms of reliability and low-cost required for CO2 capture systems. Projects for the manufacture of more suitable membrane materials for CO2 capture in large-scale applications started in mid-2000s.
3. Distillation of a liquefied gas stream and refrigerated separation
A gas can be made liquid by a series of compression, cooling and expansion steps and then the components can be separated in a distillation column. This technology was also applied to separate CO2 from other gases and to remove impurities from high purity CO2 streams.
Geological, marine and carbonate storage
The permanent and safe storage of captured CO2 is a crucially requirement to justify the high costs associated with CO2 capturing and transportation. It can be undertaken in a variety of geological settings in sedimentary basins: petroleum fields, depleted gas fields, deep coal seams and saline formations are all suitable for the so called geological storage. Basins suitable for CO2 storage have characteristics such as thick accumulations of sediments, permeable rock formations saturated with saline water (saline formations), extensive covers of low porosity rocks (acting as seals), and structural simplicity. It is also important to know how securely and for how long (decades, centuries or millennia) stored CO2 will be retained.
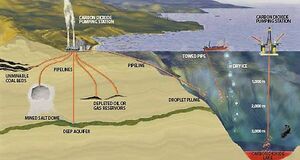
Subsurface geological storage is also achievable offshore through pipelines from the shore or from offshore platforms. The continental shelf and some adjacent deep-marine sedimentary basins are potential offshore storage sites, but the majority of sediments of the abyssal deep ocean floor are too thin and impermeable to be suitable for geological storage (Fig. 3). More recently, attention has been given to storage in caverns, basalt and organic-rich shales, but many technical aspects have to be developed.
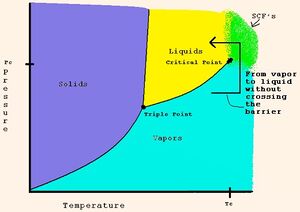
To geologically store CO2, it has to be compressed to a dense fluid state known as supercritical[14] (Fig. 4). Fluids have been injected on a massive scale into the deep subsurface for many years to dispose of unwanted chemicals, pollutants or by-products of petroleum production, and to enhance the production of petroleum and gas. Depending on the rate that temperature increases with depth (the geothermal gradient)[15], the density of CO2 will increase until at about 800 m it will reach the supercritical state. Still in early 2000s, the injection of CO2 has been done at a relatively small scale.
There are three basic types of geological formations that have adequate CO2 storage potential:
- Deep saline aquifers
- Oil and gas reservoirs
- Deep (not mineable) coal seams
- In addition, two not-subterranean promising alternatives have been explored in past 30 years:
- Seas and Oceans
- Direct Mineral Carbonation
1. Deep saline aquifers
Aquifers are strata of porous rock often containing salt water and through which liquids or gases can flow - an impermeable overlying cap-rock is normally positioned above the aquifer. The CO2 is introduced into the aquifer via a bore-hole resulting in partial dissolution of the CO2 in the salt water and partial displacement of the water by the fluid. There may also be reactions with the surrounding rock over longer periods. If the rock is a silicate, retention of the CO2 in the aquifer could be enhanced since a proportion of the gas could be bound up as a solid carbonate.
2. Oil and gas reservoirs
Storage of CO2 in partially depleted petroleum or gas fields has been considered economically convenient since the 1970s since it drives out residual oil and natural gas - extending the economic life of these fields. From the first CO2 injection experimental project in 1958 in the United States, this process has proven to be both profitable and an efficient mechanism for hydrocarbon recovery. Research needs are identical to those of CO2 storage in aquifers.
The capacity of natural gas fields to store carbon from injected CO2 is greater than the carbon content of the original natural gas by about a factor of 2, depending on the depth, pressure and temperature of the reservoir. The global available storage capacity in early 2000s was estimated to be 513 to 1,503 Gigatons of CO2 (1 gigaton equals to 1,000,000,000 metric tons). In general terms, natural gas fields are shut down when the gas pressure drops below an economically viable level (approximately 30 bar). CO2 injection increases the pressure to an acceptable level (below the original pressure, in order to retain leak-tightness). Since most gas reservoirs are more than 80 % exhausted, enhanced production is limited. The concept has not yet been fully developed, mainly due to fears that the quality of the gas would be diminished by mixing with the injected CO2. No information is available on the industrial scale recovery of natural gas by CO2 injection. Storage in petroleum fields appears to be better than in natural gas fields, because the latter are often already more than 80 % exhausted, whereas the degree of exhaustion of oil fields is generally much lower
3. Deep (not mineable) coal seams
Another economically promising method for storing CO2 is offered by deep coal seams that cannot be mined. In this case, the gas is injected into the seam via boreholes and adsorbed onto the coal. Methane is desorbed (driven out from the coal), thereby increasing the methane production rate. This recovery process is known as the ECBM method (Enhanced Coalbed Methane)[16]. The storage capacity of coal for CO2 is influenced by the permeability and porosity of the strata and fractures within the strata, which can also be caused by the drilling operations or swelling of the coal due to CO2 adsorption. Research is required to improve the understanding and modeling of the adsorption/desorption processes and the subsequent swelling of the coal in relation to the overall CO2 storage capacity.
4. Seas and Oceans
Captured CO2 can be injected into the ocean at great depth, where most of it would remain isolated from the atmosphere for centuries. There have been small-scale field experiments and 25 years of theoretical, laboratory, and modelling studies of intentional ocean storage of CO2, but ocean storage has not yet been deployed or thoroughly tested. CO2 to be retained in the sea over centuries has to be introduced below the so-called thermocline - the limit between the well-intermingled water near the surface and the cold, deeper layers. This process is considered to be not allowed under current international conventions and the impact on the marine life cycle is not fully understood.
5. Direct Mineral Carbonation
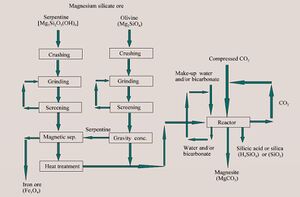
Direct mineral carbonation has been investigated as a process to convert gaseous CO2 into a geologically stable, solid final form (Fig. 5). The process utilizes a solution of sodium bicarbonate, sodium chloride, and water, mixed with a mineral reactant, such as olivine or serpentine. Carbon dioxide is dissolved into this slurry, by diffusion through the surface and gas dispersion within the aqueous phase. The process includes dissolution of the mineral and precipitation of magnesium carbonate (MgCO3) in a single unit operation. Early studies suggested that the mineral dissolution rate is primarily surface controlled, while the carbonate precipitation rate is primarily dependent on the bicarbonate concentration of the slurry. Current studies include further examination of the reaction pathways, and an evaluation of the resource potential for the magnesium silicate reactant, particularly olivine. The storage of CO2 in carbonates will be investigated further as part of the Zero Emission Coal Alliance (ZECA).[17]
Limitations and future challenges
For CO2 capture from industrial processes, a number of technologies that are commonly used in natural gas sweetening and ammonia production are already used on a commercial scale. For other types of industrial systems capturing CO2 from steel and cement production, further work is still needed. Capture processes reliant on post-combustion capture or oxy-fuel combustion are less developed or are available at a smaller scale. For pre-combustion capture many of the required systems have been developed and applied in industry already.
Although many of the technologies required for CO2 capture in post-combustion, pre-combustion and oxy-fuel combustion are well known, gaps in knowledge are in the practical and/or commercial demonstration of capture integrated systems. This would be essential to minimize and establish with certain the cost of CO2 capture and its use on a large scale, particularly in power generation applications, but also for cement, steel and other large industries. Operating experience is also needed to test system reliability, improved methods of system integration, methods to reduce the energy requirements for CO2 capture, improved process control strategies, and the use of optimized functional materials for the implementation of capture processes with advanced, higher efficiency power cycles.
Conclusion
CO2 geological storage has become a strand of scientific literature and discussions in the fields of energy and environmental policy. This has been happening very recently, but with an exponentially increasing weight from month to month due to its extraordinary potential for reducing greenhouse emissions, together with "capture" techniques, so as to be defined together as CCS technologies= Capture & Storage of CO2.
The Third Assessment Report (TAR)[18] stated that no single technology will provide the emission reductions needed to achieve substantial results, and only the convergence of mitigation measures will be really effective. Other mitigation options include energy efficiency improvements, the switch to less carbon-intensive fuels, nuclear fusion,[19] renewable energy sources, enhancement of biological sinks, and reduction of non-CO2 greenhouse gas emissions.
Envisioning methods for CO2 capture will occupy the next several decades of engineers, chemists, physicists, earth scientists, and mathematicians. CO2 capture is often, erroneously, considered in the context of point sources such as coal-fired power plants or industrial processes, but breakthroughs in research must lead to applications of CO2 capture to a wider realization of its abatement potential. Process engineering, materials science, catalysis and nanoscience will likely play key roles in hybridizing the known technologies toward an integrative approach to meet this goal.
CCS techniques were emphasized during the Green House Gas Technologies (GHGT)[20] international conferences, starting especially with the Fifth meeting held in Australia in 2000, then with growing scientific enthusiasm in the Sixth meeting held in Kyoto, Japan, in October. 2002: it was at the GHGT-6 international conference that had been established that the geological storage [21] of CO2 is a new interdisciplinary science, a priority for the non-postponable resolution of the growing greenhouse emissions into the atmosphere.
The widespread application of CCS would depend on technical maturity, costs, overall potential, diffusion and transfer of the technology to developing countries and their capacity to apply the technology, regulatory aspects, environmental issues and public perception. A new global interest in extending CO2 capture to power plants is producing a dramatic expansion in R&D and many new concepts associated with clean energy conversion processes (FIG. 6). The application of CO2 capture technologies beyond concentrated sources is in view, but less tractable. The first and second laws of thermodynamics[22] set boundaries on the minimum work required for CO2 separation. Real separation processes will come with irreversibilities and subsequent inefficiencies taking researchers further from best-case scenarios. The inefficiency of a given process reveals itself in the form of operating and maintenance, and capital costs.
List of the most common acronyms used in the CO2 geological storage sector
- ASU: Air Separation Unit
- ATR: Auto Thermal Reforming: a process in which the heat for the reaction of CH4 with steam is generated by partial oxidation of CH4.
- AZEP: Advanced Zero Emission Plant
- BAT: Best Available Technique
- BECCS: Bioenergy with CCS
- BEP: Best Environmental Practice
- BGR: Federal Office for Geo-sciences and Raw Materials (Germany)
- BOA: Innovative Technology for Lignite-fired Power Plants
- BPEO: Best Practical Environmental Option
- CAR: Ceramic Auto-thermal Recovery
- CBM: Coal bed methane
- CC: Combined Cycle
- CCS: Carbon dioxide capture and storage
- CCUS: Carbon Capture Utilisation and Storage
- CDM: Clean development mechanism: a Kyoto Protocol mechanism to assist non-Annex 1 countries to contribute to the objectives of the Protocol and help Annex I countries to meet their commitments.
- CENS: CO2 for EOR in the North Sea
- CFB: Circulating Fluidised Bed
- CLC: Chemical Looping Combustion
- CMB: Circulating Moving Bed
- CO2 A (avoided): The difference between CO2 captured, transmitted and/or stored, and the amount of CO2 generated by a system without capture, net of the emissions not captured by a system with CO2 capture
- CO2 E (equivalent): A measure used to compare emissions of different greenhouse gases based on their global warming potential
- COE: Cost of electricity
- CSIRO: Commonwealth Scientific and Industrial Research Organisation (Australia)
- CTH: Chalmers Technical High School
- D: Darcy, a non-SI unit of permeability, abbreviated D, and approximately =1μm2
- DAC: Direct Air Capture
- DACCS: Direct Air Capture with Carbon Storage
- DEA: Diethanolamine
- DIC: Dissolved Inorganic Carbon.
- DMPEG: Dimethylether-polyethylene-glycol
- DOC: Dissolved organic carbon
- DSR: Discovered Storage Resources. The estimated quantity of Total Storage Resources, as of a given date, in which the potential for storage has been ascertained within an assessed geologic formation.
- DTI: Department of Trade and Industry (UK)
- ECBM: Enhanced coal bed methane recovery; the use of CO2 to enhance the recovery of the methane present in unminable coal beds through the preferential adsorption of CO2 on coal.
- ECCP: European Climate Change Programme
- EGR: Enhanced gas recovery: the recovery of gas additional to that produced naturally by fluid injection or other means
- EOR: Enhanced oil recovery: the recovery of oil additional to that produced naturally by fluid injection or other means
- ESG: Environmental, Social and Corporate Governance
- EU FVI: European Commission Framework VI R&D Programme
- FBC: Fluidized bed combustion: combustion in a fluidized bed
- FEED: Front-End Engineering Design
- FGD: Flue Gas Desulphurisation
- GEO-SEQ: Geological Sequestration of CO2
- GESTCO: Geological Storage of CO2
- GHG: Greenhouse gases: carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), hydroflurocarbons (HFCs), perfluorocarbons (PFCs), and sulphur hexafluoride (SF6)
- GT: Gas Turbine
- Gt: Gigatonne
- Gw: Gigawatt
- HAZOP: HAZard and OPerability, a process used to assess the risks of operating potentially hazardous equipment
- HHV: High Heating Value: the energy released from the combustion of a fuel that includes the latent heat of water
- HRSG: Heat Recovery Steam Generator
- HTW: Hochtemperatur (High Temperature) Winkler Process
- IEA: International Energy Agency
- IEA GHG: International Energy Agency, Greenhouse Gas Research & Development Programme
- IGCC: Integrated Gasification Combined Cycle: power generation in which hydrocarbons or coal are gasified (q.v.) and the gas is used as a fuel to drive both a gas and a steam turbine
- ISR: Inaccessible Storage Resources. The estimated portion of Discovered or Undiscovered Storage Resources, as of a given date (i.e., the time of the evaluation), that are not usable by future storage development projects.
- IP: Intermediate Pressure
- IPCC: Intergovernmental Panel on Climate Change
- IPPC: Integrated Pollution Prevention and Control
- JI: Joint Implementation: under the Kyoto Protocol, it allows a Party with a GHG emission target to receive credits from other parties.
- LCFS: Low Carbon Fuel Standard
- LHV: Lower heating value: energy released from the combustion of a fuel that excludes the latent heat of water.
- LNG: Liquefied natural gas
- LP: Low Pressure
- LPG: Liquefied Petroleum Gas
- MBHX: Moving Bed Heat Exchanger
- MCFC: Molten Carbonate Fuell Cell
- MDEA: Methyldiethanolamine
- MEA: Monoethanolamine
- MMV: Monitoring, Measurement and Verification
- Mt: Million Metric Tonnes
- MWh: Megawatt-hour
- NAP: National Allocation Plan
- NDC: Nationally Determined Contribution
- NG: Natural Gas
- NGCC: Natural gas combined cycle: natural-gas-fired power plant with gas and steam turbines
- NMP: N-methyl-2-pyrrolidon
- NPV: Net present value: the value of future cash flows discounted to the present at a defined rate of interest.
- O&M: Operation and Maintenance
- OECD: Organization for Economic Co-operation and Development
- OSPAR: Convention for the Protection of the Marine Environment of the North-East Atlantic, which was adopted at Paris on 22 September 1992.
- PAFC: Phosphoric Acid Fuel Cell
- PC: Pulverized Coal
- pCO2: The partial pressure (q.v.) of CO2
- PEM: Polymer Electrolyte Membrane
- PF: Pulverized Fuel
- PSA: Pressure swing adsorption: a method of separating gases using the physical adsorption of one gas at high pressure and releasing it at low pressure
- R&D: Research and Development
- RITE: Research Institute of Innovative Technology for the Earth (Japan)[23]
- SC: Storage Capacity. Those quantities of Total Storage Resources anticipated to be commercially accessible in the characterized geologic formation by application of development projects from a given date forward under defined conditions.
- SCFs: Supercritical fluids
- SACS: Sleipner Aquifer for CO2 Storage
- SCR: Selective catalytic reduction
- SDS: Sustainable Development Scenario
- SINTEF: Foundation for Scientific and Industrial Research at the Norwegian University of Science and Technology
- SMR: Steam methane reforming: a catalytic process in which methane reacts with steam to produce a mixture of H2, CO and CO2
- SNG: Synthetic natural gas: fuel gas with a high concentration of methane produced from coal or heavy hydrocarbons
- SOE: State Owned Enterprise
- SOFC: Solid oxide fuel cell: a fuel cell in which the electrolyte is a solid ceramic composed of calcium- or yttrium-stabilized zirconium oxides
- SRES: Special Report on Emissions Scenarios; used as a basis for the climate projections in the TAR
- SUM: Sum of Storage Resources. Is not part of the SPE-SRMS but is used to show all the known quantities of storage resources estimated in a geologic formations.
- TAR: Third Assessment Report of the Intergovernmental Panel on Climate Change
- TCR: Total capital requirement
- TNO: The Netherlands Organisation for Applied Scientific Research
- TWH: Terrawatt Hour
- UNCLOS: United Nations Convention on the Law of the Sea, which was adopted at Montego Bay on 10 December 1982.
- UNFCCC: United Nations Framework Convention on Climate Change, which was adopted at New York on 9 May 1992
- US DOE: United States Department of Energy
- USR: Undiscovered Storage Resources. The estimated quantity of Total Storage Resources, as of a given date, in which the suitability for storage has not been ascertained within the target geologic formation.
- X (well class): A regulatory classification for wells used for the injection of fluids into the ground
- ZECA: Zero Emission Coal Alliance
See also
- Glossary of the Technical Terminology Used in the Petroleum Industry, 1890 - 1950
- Fossil fuels
- Biofuels
- Synthetic Fuels
References
AA.VV. 2004. CO2 Capture and Storage. A VGB Report on the State of the Art. Essen: VGB PowerTech e.V.
van Dijk, J.P. “Lo stoccaggio della CO2 nel sottosuolo. Il ruolo delle Geoscienze”. Atti del 1º Congresso dell’Ordine dei Geologi di Basilicata, ‘Ricerca, Sviluppo ed Utilizzo delle Fonti Fossili: Il Ruolo del Geologo’, Potenza, 30 Novembre - 2 Dicembre 2012.
Gluyas, J. G., and Simon A. Mathias. 2013. Geological storage of carbon dioxide (CO₂): geoscience, technologies, environmental aspects and legal frameworks. Cambridge: Woodhead Publishing Limited
Herzhog, H. 2000. “The economics of CO2 separation and capture”. Technology 7, no. 1: 13-23.
Herzhog, H. 2001. “What future for carbon capture and sequestration?” Environmental Science and Technology 35, No. 7: 148-153.
Herzhog, H., and Colomb, D. 2004. “Carbon capture and storage from fossil fuel use”. Encyclopedia of Energy, C.J. Cleveland Edition: 277-287.
Houghton, J. T., Intergovernmental Panel on Climate Change. Working Group I (Eds). 2001. Climate change 2001: the scientific basis. Cambridge, UK: Cambridge University Press.
Metz, Bert, Ogunlade R Davidson; Heleen De Coninck; Manuela Loos; Leo Meyer, Intergovernmental Panel on Climate Change. Working Group III. 2005. IPCC special report on carbon dioxide capture and storage. Cambridge: Cambridge University Press for the Intergovernmental Panel on Climate Change.
Miller, S.A., Collettini, C., Chiaraluce, L., Cocco, M., Barchi, M. and Kaus, B.J., 2004. “Aftershocks driven by a high-pressure CO2 source at depth”. Nature 427: 724-727.
W.K. O’Connor, D. C. Dahlin, D.N. Nilsen, G.E. Rush, R.P. Walters, and P.C. Turner. 2001. Carbon Dioxide Sequestration by Direct Mineral Carbonation: Results from Recent Studies and Current Status. Albany, Oregon: Albany Research Center, Office of Fossil Energy, U.S. Dept. of Energy.
Quattrocchi, Fedora, Bencini, Roberto. 2015. “Lo stoccaggio geologico di CO2: stato dell’arte in Italia ed all’estero”. La Termotecnica, Maggio 2015: 39-46.
Quattrocchi, F., Bencini, R., Baubien, S., Cinti, D., Cardellini, C., Galli, G., Lombardi, S., Pizzino, L. and Voltattorni, N., 2003. “Geological sequestration of industrial CO2: approaches and perspectives”. In: Proceedings of the Workshop on European Strategies on climate change: options and strategies for the future. Semester of Italian EU Presidency. Meeting, Firenze, Fortezza da Basso, 11-12 September 2003.
Stranges, Anthony N. Pioneering Studies on Acid Rain. ICOHTEC 2020 DIGITAL, July 15-17. Symposium 1.4 “Energy and the Environment”. Conference Paper.
Wulff, Petter. 2020. The Climate Legacy of Arrhenius. ICOHTEC 2020 DIGITAL, July 15-17. Symposium 1.4 “Energy and the Environment”. Conference Paper.
Further Reading
- ↑ https://www.britannica.com/science/carbon-dioxide
- ↑ https://www.energy.gov/fe/science-innovation/oil-gas-research/enhanced-oil-recovery
- ↑ https://www.bgs.ac.uk/
- ↑ https://www.equinor.com/en.html
- ↑ https://eng.geus.dk/
- ↑ https://www.imperial.ac.uk/
- ↑ https://www.bp.com/
- ↑ https://www.tno.nl/en/
- ↑ http://large.stanford.edu/publications/coal/references/leep/docs/chapter2.pdf
- ↑ https://ieaghg.org/docs/oxyfuel/w1/04W1Wall.pdf
- ↑ https://netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/syngas-composition
- ↑ https://www.osakagas.co.jp/en/rd/fuelcell/pefc/reformed/shift.html
- ↑ https://www.nature.com/articles/s41598-019-57151-x
- ↑ http://www.supercriticalfluid.org/Supercritical-fluids.146.0.html
- ↑ https://www.science.gov/topicpages/b/background+geothermal+gradient
- ↑ https://usea.org/sites/default/files/media/Potential%20for%20Enhanced%20coalbed%20methane%20recovery%20-ccc252.pdf
- ↑ https://www.osti.gov/biblio/772614-overview-zeca-zero-emission-coal-alliance-technology
- ↑ https://www.ipcc.ch/site/assets/uploads/2018/03/WGI_TAR_full_report.pdf
- ↑ https://courses.lumenlearning.com/boundless-chemistry/chapter/nuclear-fusion/
- ↑ https://ghgt.info/
- ↑ https://www-sciencedirect-com.ezproxy.lib.ou.edu/book/9780080442761/greenhouse-gas-control-technologies-6th-international-conference
- ↑ https://www.grc.nasa.gov/www/k-12/airplane/thermo.html
- ↑ http://www.rite.or.jp/en/