Octane Number
The original version of this article was created by Francesco Gerali, 2020 Elizabeth & Emerson Pugh Scholar in Residence at the IEEE History Center
It is recommended this article be cited as:
F. Gerali (2020). Octane Number, Engineering and Technology History Wiki. [Online] Available: https://ethw.org/Octane_Number
Anti-detonating, or anti-knocking, quality is a pivotal property of gasoline that depends by the octanes number. Chemical engineers in refineries focused research efforts on that quality since the beginning of automotive industry boom in the 1910s. When detonation occurs, engines emit a characteristic “flicker” metallic sound, which is due to the partial combustion of the liquid fuel. This resulted in loss of power, lower performance, dangerous engine overheating, and premature wear of pistons and piston rings. Detonation was a harmful mechanical deficiency for car engines (especially more disastrous for airplanes since military and civil aviation relied almost entirely on propeller engines until the 1950s) because the rapid rise in temperature may cause expansion, or "freezing," of cylinder heads and pistons, thus disabling the motor.
The other important interdependent variable in the knocking effect is the engine compression ratio. It expresses the ratio between the maximum and minimum amount of space which gases occupy in the cylinder after and before explosion. For instance, if the space taken up by the gases after the explosion is six times that before the explosion, the compression ratio of the engine is 6:1. Gasoline begins to knock, or detonate, when the compression ratio of the engine exceeds certain limits. These limits are different for different gasolines even if the two gasolines are similar in most of their physical properties. Detonation may be avoided by employing engines having low compression ratios, but this was never considered a convenient solution. High compression ratios save gasoline consumption and allow constructors to forge engine blocks with lighter metallic leagues.
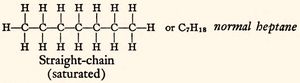
The antiknock characteristics of gasoline are expressed in terms of octane numbers. The octane number refers to standard mixtures of two substances. One of these is the isooctane,[1] more precisely the isomer of octane, 2,2,4-trimethylpentane;[2] the other is heptane.[3] Isooctane has exceptional antiknocking characteristics and petroleum chemists classified it with the arbitrary octane number of 100. On the contrary, heptane detonates much more easily, and its assigned octane number is zero. Blending these two chemical in different proportions it is possible to obtain a vast array of fuels that cover the whole scale of possible gasoline mixtures. This scale is used as a standard tool of reference to classify gasoline-like fuels. It follows that, for example, an octane number of 70 corresponds to a mix containing 70% by volume of isooctane and 30% by volume of heptane.
References
Further Reading
Bell, H. S. 1930. American Petroleum Refining. New York: Van Nostrand.
Brooks, B. T., Dunstan, A. E., Nash, A. W., and Tizard, H. T. 1938. The Science of Petroleum. London: Oxford University Press.
Nelson, W. L. 1941. Petroleum Refinery Engineering. New York, London: McGraw-Hill Book Company.
Society of Automotive Engineers. 1976. A History of the automotive internal combustion engine. Warrendale, Pa: Society of Automotive Engineers.