First-Hand:Applying digital television technology to medical imaging = x-ray dosage reduction and non-invasive angiography
Contributed by Stanley Baron, IEEE Life Fellow
Introduction
I began working on the application of digital technology to television starting in 1962 and from that time on, worked with other individuals in Europe and Asia, as well as America, on advancing the technology.
From 1979 through 1985, I participated in a research project on behalf of my employer, Thomson-CSF Laboratories in Stamford, Connecticut in cooperation with the Lasseret i Lund (University Hospital in Lund, Sweden) and the University of Utah Medical Center in Salt Lake City, Utah. The project involved the application of digital filtering to fluoroscopic images.
X-ray dosage reduction
The Swedish medical system had noted that children born with heart defects were observed about a decade later to exhibit abnormally high rates of carcinoma. Evidence indicated the high incidence of cancer was due to imaging radiation exposure when monitoring their condition as infants. Dr. Gudmond Svahn of the University of Lund was investigating methods for reducing imaging radiation exposure. He asked whether a real-time digital processor could be designed that would permit the use of lower radiation dosage levels while retaining fluoroscopic image quality.
When radiation levels are lowered, fewer photons are generated during a fluoroscopic study, resulting in noisier images. I was approached by the Swedes, and based on my knowledge of noise reduction techniques employed for improving television images, I designed a first-order recursive filter device specifically designed for removing noise and improving fluoroscopic images under low-radiation conditions. The processor digitized the image frames in real time (at 50 Hz or 60 Hz image picture rates) with a 15.75 MHz sample rate, which produced an orthogonal sampling structure at either picture rate. The recursive processor employed an iterative process that compared samples of the image with adjacent samples in the horizontal (x), vertical (y), and, since the memory included a frame-store, in the temporal (t) dimension, using an algorithm that modified samples that were deemed ‘noise’ and restored the missing information. Since the computational requirements were intensive, the device was designed as a dedicated, special purpose digital video processor, and was not based on a standard computer.
Dr. Svahn conducted imaging studies using the prototype device. We produced images using 50% of the normal level of contrast material injected into the blood stream and with reduced radiation levels (between 20% and 50% of normal). The resulting images as processed by the recursive filter were deemed of equal quality to those produced using full contrast and normal radiation levels. The fact that less contrast material was required was also an advantage, since the contrast material used at the time was toxic to humans. The device became the basis of a new digital signal processor (DSP) manufactured in Stamford, Connecticut and sold commercially throughout the world as the DSP 9200. We placed one machine in each of the regional medical hospitals in Sweden. The device was well received in other parts of Europe.
Non-invasive angiography
Working with Dr. Svahn and his team at Lund led to a second phase of the project. Imaging performed on young children with heart defects consists of angiographic imaging, an invasive process, which requires placing a catheter directly in the arteries leading to the heart. It was my understanding that 2% of the patients developed significant problems during this procedure, resulting in a mortality rate of 0.1%. In developing the DSP 9200, my participation in the project with Dr. Svahn involved testing the digital processor in real-life situations, and I participated in a series of angiography procedures.
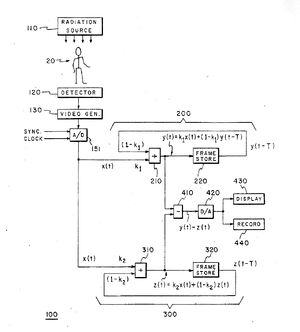
As a result of my exposure to angiography studies, I proposed to Dr. Svahn the possibility of employing a second-order recursive filter device as a band-pass filter. I drew a block diagram for Dr. Svahn and explained how it could work. In theory, this filter would allow a bolus of dye injected into the arm to be imaged as it traveled through the patient's body. Such a device could, in theory, allow sufficient enhancement of the bolus of contrast material while removing background information to support non-invasive angiographic imaging.
I suggested to Dr. Svahn that it would be helpful to work with a medical center in the United States, as well as my continuing to work with him in Sweden to develop this new device. Svahn was planning a tour of major American medical centers and promised to advise me of possible centers for partnership upon his return from the trip. Figure 1 is a photograph of Dr. Svahn and the diagram for the second-order filter I drew on the blackboard in his office prior to his trip to America. Figure 2 provides a more readable version of the same system block diagram drawn for use in the patent application.
A few weeks after arriving in the United States, Dr. Svahn called me while he was at one of his scheduled stops, the University of Utah Medical Center. He had discussions with a Dr. Robert Kruger who had a concept for non-invasive angiography. Dr. Kruger had drawn the same block diagram that I had. Kruger complained that he couldn't find anyone who could design the necessary equipment. Svahn suggested that Kruger and I get together. I flew out to Utah and held discussions with Kruger and Dr. Jim Nelson, a cardiologist who was working with Kruger. After that one visit, an agreement was drawn up between Thomson and the two universities to proceed on the development of a second-order filter.
I returned to Stamford, and our project team designed and built the second-order digital recursive filter DSP that I had diagramed for Svahn. One of the more challenging aspects of the design of the second-order filter was the creation of the controlling software that was totally crash-proof. The bulk of that effort fell on the shoulders of Tom Lewis and Marc Poncelet. We began animal studies at the University of Utah, and the technique met our expectations. Kruger and Nelson then filed an investigation-protocol proposal with the U.S. government seeking permission to begin human studies.
On the last day of one of my visits to Salt Lake City, I had a very uplifting experience involving our first human study. Dr. Nelson became aware of a patient at the hospital who we might be able to help with our new processor. She was over 70 years-of-age and was experiencing internal hemorrhaging. The staff had conducted several days of angiography studies but none of the images showed the location of the leak. In the meantime, the patient was growing weaker, and her medical team was concerned about maintaining her life. Dr. Nelson approached the patient's cardiologist and explained our system’s capabilities to her.
The cardiologist was reluctant to put her patient through a new process still under study. Dr. Nelson explained that our process was not only non-invasive, but exposed the patient to only 20% of the normal radiation and, further, required less than half the contrast material used in a normal angiography. It was my understanding that due to the toxic nature of the contrast material, normally, a patient had to have the contrast material flushed out of his or her system and then be rested for one full day after a study before another study could be performed. Dr. Nelson explained that with our system, a study could be done in the early morning and the patient would be available for a traditional study within a few hours if the use of our system didn’t locate the problem. We demonstrated the system to the cardiologist using one of the recorded animal studies. The cardiologist and her patient decided to proceed with our non-invasive approach.
I had designed into the equipment's capability a ‘memory mode’ as one of the modes of operation. This mode could display the entire trace of the contrast material's path through the body in a single image. Thus, the device offered a choice of watching the bolus of dye move through the patient's cardiovascular system or capturing all of its travel in a single image.
The patient was brought into the Medical Center imaging room in which our equipment had been installed, and the study conducted. Given the subtlety of the problem, the team decided to operate the processor in the memory mode. Twenty seconds after the procedure began; the image on the screen showed a tiny, swirling eddy of contrast at the point where a major vein met a smaller vein. The spot was identified, the patient sent to surgery, and the ‘leak’ corrected. The patient's cardiologist became one of our strongest supporters. In its first application in a human study, the device helped to save a life.
I returned to Stamford to convert the prototype design to a commercial product. I continued to journey both to Lund and Salt Lake City to conduct additional studies and perfect the algorithms. The system was programmable, in that the design provided different sets of filter parameter values for different types of angiography studies. The computer interface allowed the medical specialists to select an angiography study, such as an 'aortic arch study' or a 'peripheral study' and the processor reprogrammed itself for the proper set of filter parameter values for the selected study. During the course of this program, I participated in dozens of angiograms either at Salt Lake or in Lund.
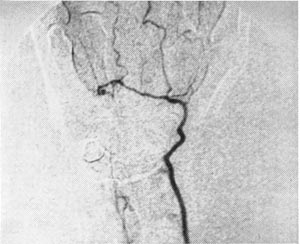
The device was sold as the DSP 9300. Figure 3 shows a non-invasive angiogram study using the DSP 9300.
As work progressed, I continued to incorporate additional features into the design, and began preparing a patent disclosure that described this latest version of the equipment. The patent application was filed with the U.S. patent office in the beginning of January 1982.
References
For those individuals interested in reading further, I offer the following references:
[1] R.McMann, S.Baron, et al, “The DFP Digital Noise Reducer, a Real-Time High Resolution, Digital Video Processing System for X-Ray Fluoroscopy,” CONFERENCE ON DIGITAL RADIOGRAPHY, S.P.I.E., September 1981.
[2] R.Kruger,...S.Baron, et al, “Optimization of Time Domain Filtering Functions for Digital Subtraction Angiography,” R.S.N.A., 67th Scientific Assembly, November 1981, (#115).
[3] R.Kruger,...S.Baron, et al, “Patient Motion Detection and Compensation in Digital Intravenous Subtraction Angiography,” R.S.N.A., 67th Scientific Assembly, November 1981, (#216)
[4] R.Kruger,...S.Baron, et al, “Vascular Tracing in Conjunction with Digital Subtraction Angiography I: Theory and System Description,” R.S.N.A., 67th Scientific Assembly, November 1981, (#385)
[5] R.Kruger,...S.Baron, et al, “Temporal Filtering Techniques for Digital Angiography,” paper presented at University of Kiel, W.Germany, May 1982.
[6] J.Nelson,...S.Baron, et al, “Clinical Applications of Digital Filtration Techniques,” paper presented at University of Kiel, W.Germany, May 1982.
[7] R.Kruger,...S.Baron, et al, “Dynamic Tomography in Real-Time Using a Temporal Filtering Technique,” R.S.N.A., 68th Scientific Assembly, November 1982, (#45).
[8] W.Bateman, R.Kruger, S.Baron, “Parametric Imaging Using a Recursive Processing System,” R.S.N.A., 68th Scientific Assembly, November 1982, (#319).
[9] S.Baron, “Non-invasive Angiography Using Real-Time Temporal Filtering,” IEEE Connecticut Section, Engineering in Medicine, 24 March 1983.
[10] R.Kruger,...S.Baron, et al, “Temporal Filtering Techniques for Digital Angiography,” DIGITAL IMAGING IN CARDIOVASCULAR RADIOLOGY, Theme-Stratton, Inc., New York, 1983, pp.80-88.
[11] J.Nelson,...S.Baron, et al. “Clinical Applications of Digital Filtration Techniques,” DIGITAL IMAGING IN CARDIOVASCULAR RADIOLOGY, Theme-Stratton, Inc., New York, 1983, pp.183-192.
[12] S.Baron, “Digital Fluoroscopy, 1st and 2nd Order Recursive Filters,” (invited paper), Seminar on Digital Radiography, University of Lund, Sweden, 12 and 13 June 1984.
Stanley Baron, F-IEEE