First-Hand:Discovery of Superconductivity at 93 K in YBCO: The View from Ground Zero: Difference between revisions
m (→Since 1989) |
|||
| Line 782: | Line 782: | ||
</blockquote> | </blockquote> | ||
The final hearing for the patent case is conducted during the summer of 1998, by my recollection. According to the patent attorney at the time, Steve Kelber, the hearing went very well,<ref name="ftn165">While the UAH team had not yet found Chu’s “later papers” from the mid-’90s, we did have Chu’s ''Novel Superconductivity'' paper (to be discussed in greater detail in Part II) that established the date of the discovery as 29 January 1987, contrary to the 1 February discovery story Houston presents in their side of the case. As communicated to me by Kelber, that paper was introduced into the final hearing (over multiple objections by the Houston attorneys asserting the long-since closure of the discovery period). All objections were overruled, and it seemed that a key leg of the Houston account had been destroyed. However, because that paper was not in the written record and there was no transcript taken, the two “replacement judges” could not benefit by it.</ref> and he was cautiously optimistic of a victory. The final decision uncharacteristically takes almost six months. According to Kelber, one of the three judges had quit and another retired in the period before contributing to the final decision. | The final hearing for the patent case is conducted during the summer of 1998, by my recollection. According to the patent attorney at the time, Steve Kelber, the hearing went very well,<ref name="ftn165">While the UAH team had not yet found Chu’s “later papers” from the mid-’90s, we did have Chu’s ''Novel Superconductivity'' paper (to be discussed in greater detail in Part II) that established the date of the discovery as 29 January 1987, contrary to the 1 February discovery story Houston presents in their side of the case. As communicated to me by Kelber, that paper was introduced into the final hearing (over multiple objections by the Houston attorneys asserting the long-since closure of the discovery period). All objections were overruled, and it seemed that a key leg of the Houston account had been destroyed. However, because that paper was not in the written record and there was no transcript taken, the two “replacement judges” could not benefit by it.</ref> and he was cautiously optimistic of a victory. The final decision uncharacteristically takes almost six months. According to Kelber, one of the three judges had quit and another retired in the period before contributing to the final decision. No transcript is taken during the final hearing (an option at that time and chosen to manage costs). Thus, the two replacement judges are left with only the written record. | ||
In early 1999, I receive a phone call from one of the staff attorneys from UAH informing me that the patent court had decided in the favor of the senior party Houston based upon the conclusion that the oxygen content of our samples fell outside of the scope of the application. My immediate response was, “Are you sure you have the right patent?” New to the case, the replacement judges ignored all of the mountains of documents and based their final decision on a completely uncontested issue, failing to understand that the oxygen ratio was essentially an unknown quantity that was only estimated and was fixed by the processing conditions. Examples in the Huntsville notes where the oxygen ratio was simply omitted as shorthand (and one ill-placed typo in my declaration) prompted the decision that we had made, quite impossibly in air or oxygen, Y<sub>1.2</sub>Ba<sub>0.8</sub>CuO<sub>1</sub>. The third judge consents but writes separately, inviting a request for reconsideration (essentially a “mini-appeal”). | In early 1999, I receive a phone call from one of the staff attorneys from UAH informing me that the patent court had decided in the favor of the senior party Houston based upon the conclusion that the oxygen content of our samples fell outside of the scope of the application. My immediate response was, “Are you sure you have the right patent?” New to the case, the replacement judges ignored all of the mountains of documents and based their final decision on a completely uncontested issue, failing to understand that the oxygen ratio was essentially an unknown quantity that was only estimated and was fixed by the processing conditions. Examples in the Huntsville notes where the oxygen ratio was simply omitted as shorthand (and one ill-placed typo in my declaration) prompted the decision that we had made, quite impossibly in air or oxygen, Y<sub>1.2</sub>Ba<sub>0.8</sub>CuO<sub>1</sub>. The third judge consents but writes separately, inviting a request for reconsideration (essentially a “mini-appeal”). | ||
Revision as of 22:06, 27 May 2016
Disclaimer
The information given below represents the best recollections, analysis, and interpretations of the author. The reader is strongly urged to examine the great volume of source materials, especially the many items that express conflicting views and opinions, and formulate his own conclusions.
Acknowledgments
I wish to acknowledge the efforts of many who have helped make this narrative possible. First, thank you to my wonderful wife Greta who has patiently kept so many balls in the air as I have had to divert so much time and energy to this endeavor. Secondly, I must express my deepest gratitude to my courageous proofreaders – Katrina Collins, Keith Parker, Brenda Wade, Barrion Palmer, David Slaton, and Bill Kuhn. Finally, many thanks must go to David Burger and IEEE for their willingness and ability to take my story public.
Dedication
To students everywhere whose achievements may have been untimely.
Preface
This narrative is a necessary blend of non-technical and somewhat technical material. I would recommend that the non-technical reader simply skim the more technical sections. The more scientifically/mathematically-inclined person is encouraged to check my work. I must apologize to the reader for my difficulties in maintaining a consistent verb tense. The telling of this story requires frequent transitions forward and backward in time, making it challenging to maintain a consistent time reference. I must also apologize for frequent repeated cross-references; the web of confusion I will attempt to unravel is simply not conducive to a serial presentation.
Introduction
In February of 1987, scientists at the University of Houston and The University of Alabama in Huntsville jointly announced the discovery of superconductivity above 90 K[1] in a material consisting of yttrium, barium, copper, and oxygen (YBCO[2]). Thirteen years later, Dr. Robert Cava of Princeton University, who has worked with the copper oxide superconductors since 1986 (then with Bell Labs), offered one of the more candid retrospectives of that event and the environment surrounding it:[3]
The excitement that electrified the physical science community after the discovery of superconductivity above the temperature of liquid nitrogen in early 1987 spread into the popular press and from there to the public at large. There were many newspaper stories, TV news reports, and several best-selling books written about the events surrounding the initial discoveries. These popularizations made some into heroes, some into villains, some minor players into geniuses, and some major players invisible. None of them ever matched my view of what actually happened. The accounts in this country seemed always to be a blend of the actual facts with American cultural values and stereotypes – such as our national pride, our desire to root for the underdog, and our need to associate remarkable historical events with the great deeds of individuals rather than the collective efforts of many different people.
…the event that attracted everyone’s attention [came when] the story began to circulate that M. K. Wu at the University of Alabama [in Huntsville] and Paul Chu at the University of Houston (Wu was one of Chu’s former students) had found a superconducting copper oxide with a transition temperature of 90 K…
As of the time of this writing [2000], more than a dozen years after the determination of its composition and the filing of the patent applications, the U.S. Patent Office is yet to determine who rightfully holds the patent to this material. Aside from the ancient Zen question: “What is the sound of one hand clapping?” no other single question, it seems, has ever been more difficult to answer.
For those at least marginally familiar with some version of the YBCO discovery story, it will quickly become obvious that what I relate below has very little in common with the most widely disseminated accounts (perhaps consistent with Cava’s sentiment). Confined to the events having a causal relationship to finding the YBCO superconductor, the true story is, in fact, not particularly difficult to tell, as causality constrains us largely to a two-month period. It also can (and will) be corroborated with great precision by the evidence, and, unlike most accounts, is not one of brilliant scientists or their insightful vision (again in line with Cava’s perspective). The “science” (if one can call it that) is dirty and ugly, fraught with mistakes, blunders, and failures – many of them, which are, in my understanding, the basis on which success is often achieved.
The principal characters in this story are Paul Chu (University of Houston), M. K. Wu (UAH and former student of Chu), and myself, Jim Ashburn (UAH, Wu’s student at the time the discovery was made). Chu, by virtue of his position, has enjoyed many more forums for his oration than have I. His stories, at least with regard to the key events along the critical path to the discovery, have virtually nothing in common with my own. Wu’s story has rarely surpassed a couple of sentences in scope and has appeared to me to be an attempt to close the gap between Chu’s story and mine. All three perspectives and more will be addressed in this narrative in varying degrees. Other characters in the story such as Ruling Meng (Houston), Pei-Herng Hor (Houston), and C. J. Torng (Huntsville) will be introduced as the narrative warrants.

A photo from the fall of 1987 showing from left to right: Pei-Herng Hor (Houston), Ruling Meng (Houston), Laura Greene (AT&T Bell Labs), Jim Ashburn (Huntsville), Maw-Kuen Wu (Huntsville), and Ching-Wu “Paul” Chu (Houston).
As stories are told and retold, the telling and retelling itself can become its own fascinating tale, and such is the case here. The first part of this narrative will cover the events leading up to the discovery, the discovery itself, and to a lesser degree (as succinctly as I am able) the events leading up to the time of this writing. The second part is a closer examination of the “retellings,” many of which I will have introduced in the first part. Having collected so much material for so long, I have been rather snidely called by at least one critic YBCO’s “self-appointed curator.” That assessment is perhaps even true, but the position is only held reluctantly, and this narrative represents my best and most serious attempt to relay the baton.
Part I, The Discovery of Superconductivity in YBCO
Liquid nitrogen-cooled superconductor.[4] It is hard to say when this term might have first been used, only slightly less difficult perhaps to identify when it first appeared in print, and there is little doubt that the idea predated the reality by decades. Recall that superconductivity was an unexpected spoil on the quest for absolute zero, with the milestones along the way being the liquefaction of common gases – oxygen (90 K), nitrogen (77 K), hydrogen (20 K), and finally helium (4 K). Ironically, it has been some of these same temperatures in reverse that became the more prominent markers on the path towards making superconductivity more practical. For seventy-five years liquid nitrogen’s affordability and efficiency as a refrigerant had loomed as a seemingly unreachable goal, but thanks to the work of an insightful pair of scientists at IBM Zurich, 1986 would climax with the impossible seemingly within reach. On June 1st of that year, a paper appeared with little fanfare in the journal Zeitschrift für Physik by the title “Possible high Tc superconductivity in the Ba-La-Cu-O system,” documenting evidence for superconductivity at an unprecedented 30 K.
Far from Zurich, the summer of 1986 was a quiet one at the Physics Department of the University of Alabama in Huntsville. I was working there in the superconductivity lab with junior physics professor M. K. Wu and had just submitted my paperwork for the physical exam required to fly on NASA’s KC-135 (the “Vomit Comet”).[5] Our lab had been flying packages on that aircraft, exploiting the microgravity conditions to increase the critical temperature (or Tc, the temperature below which a given material will superconduct) of various superconducting alloys. The graduate student who had previously been tending to the experiments found the experience quite literally nauseating, and I was slated to replace her. My plans, however, would never come to fruition.
With the start of the fall semester, Wu hired another graduate student, Chuan-Jue Torng, to help with the experiments.[6] “C. J.,” as he was known then (he now goes by the name “Terry”), was newly arrived from Taiwan. His English was a bit rough the first year, but he still quickly learned the routine of our lab.
December 1987
Wu spent the first week of December 1987 at the fall Materials Research Society (MRS) Meeting in Boston, a semiannual event we rarely missed. It is my understanding that on the previous Friday, Wu’s former graduate advisor, Ching-Wu “Paul” Chu, from the University of Houston, along with a group from the University of Tokyo led by Koichi Kitazawa, independently presented results confirming the recent observations by Bednorz and Müller – possible superconductivity around 30 K in an oxide of lanthanum, barium, and copper (LBCO).[7] [8] Kitazawa further revealed that his group had isolated the 30 K superconductivity to the barium-variant of a series of phases[9] (often called “double perovskites” at the time) first characterized in the early ‘80s by French chemists Claude Michel and Bernard Raveau.
By Chu’s accounts, he had called Wu over the weekend following the meeting, asking him to help track down a paper by the French group, presumably among the ones cited in the original Bednorz-Müller paper (either reference #16 or #21). Wu located the paper at the Redstone Scientific Information Center library[10] on the following Monday and immediately faxed it to Chu in Houston. By my recollection, Wu also retrieved on the same trip several related papers (including others by the Michel-Raveau team[11]) that I presume were also faxed. Copies that were given to me about the same time are still in my files as of this writing.
Among the titles were these:
C. Michel, B. Raveau, “Oxygen intercalation in mixed valence copper oxides related to the perovskites,” Revue de chimie minérale 21.4 (1984): 407-425 (reference #16 in the Bednorz-Müller paper).
N. Nguyen, J. Choisnet, J. Hervieu, B. Raveau, “Oxygen defect K2NiF4-type oxides: The compounds La2-xSrxCuO4-x/2+d,” Journal of Solid State Chemistry 39.1 (August 1981): 120-127.
N. Nguyen, F. Studer, B. Raveau, “Oxydes ternaires de cuivre a valence mixte de type K2NiF4 deficitaires en oxygene: Evolution progressive d’un etat semi-conducteur vers un etat semi-metallique des oxides La2-xSrxCuO4-x/2+d,” Journal of Physics and Chemistry of Solids 44.5 (1983): 389-400.
L. Er-Rakho, C. Michel, J. Provost, B. Raveau. “The Oxides La3-xLnxBa3Cu(II)5-2yCu(III)1+2yO14+y.” Journal of Solid State Chemistry 37 (1981): 151-156.
The first paper covered the phase to which Kitazawa had attributed the superconductivity in LBCO as well as its strontium and calcium cousins, in the abstract identified this way: La2−xAxCuO4−x/2+δ (A=Ca, Sr, Ba). Chu would later claim that the strontium substitution was his independently-conceived idea,[12] although both it and calcium were obvious candidates well before we had faxed the papers to Houston.[13] The second and third papers specifically focused on the strontium variant. In hindsight, the French group may have missed a Nobel Prize by simply failing to run their resistivity tests to lower temperatures. This is essentially the first of many near misses to be described in this narrative.
The fourth paper, which I will later identify as the “Er-Rakho et al. paper,” specifically describes substitutions of yttrium for lanthanum in a compound of lanthanum, barium, copper, and oxygen.[14] Chu will later claim to have independently conceived this substitution as well (a topic to be discussed ad nauseam in the pages to follow).
At least for a while, it would not be necessary in Huntsville to dream up new candidate materials, as the work by the French chemists included their examination of the electrical conductivity (down to 77 K) of a host of different compounds. Clearly, each showing metallic behavior[15] was a strong candidate for superconductivity.
I feel compelled to interject here that I have no illusions about the inevitability of the YBCO discovery; after the Bednorz-Müller announcement, it was indeed only a matter of time. If not at Alabama, then perhaps at Bell Labs, or Bellcore, or Tokyo, or elsewhere within weeks, an opinion I believe would be shared by the vast majority of researchers in the field.[16]
Concurrent with gathering some of the necessary reagents and additional equipment to work with the new materials, Wu, C. J., and I spent the remainder of the week on measurements of samples from our ongoing research. The previous two years had been devoted to studies of superconductivity in the immiscible alloys Ga-Bi and Al-In-Sn processed in microgravity conditions. It seems ironic now that we had previously been studying ways to turn one 7.7 K superconductor into an 8.3 K superconductor and another 6.5 K superconductor into a 7.4 K superconductor.
Designed for low melting point alloys, the furnaces we had used for our microgravity studies would be woefully inadequate for the copper oxides.[17] To mitigate this limitation, Professor Jack Davis, whose office was just across the hall from Wu’s, made available a furnace of his. Its capabilities would be marginal. An old but rugged piece of equipment housed in a stainless steel cabinet, the analog controller topped out at three digits – 999 °C. It would be several months before I would learn the significance of this constraint. Davis’s lab was also home to a second furnace being used by Jason Kinzer,[18] a friend of mine and graduate student of Professor Elmer Anderson.[19] Jason was experimenting with vapor growth of zinc selenide crystals and would occasionally offer us the use of his furnace, which was capable of temperatures around 1200 °C. By the end of the second week of December, the preparation of our first copper oxide sample was under way using simple solid-state reaction[20] of oxides and carbonates.[21]

A few of the reagents salvaged from the UAH superconductivity lab.
When I first joined Wu, he had been recording results from his AC resistivity and magnetic susceptibility tests on a plotter. Using two low voltage supplies, input signals to the plotter would be offset so that higher sensitivities could be used. The plotter data would then be read manually (one of my jobs, of course) and then biased, scaled, and translated as necessary.[22] Typically, no more than 40 to 50 points would be obtained for each test to be entered into a computer by hand for charting. Having grown very weary of this chore, I seized an opportunity when Wu brought a Hyundai personal computer in the lab. I soon had the computer interfaced with the lock-in amplifier via its RS-232 interface, an A/D card receiving the thermocouple and germanium thermometer signals, and custom GW-BASIC programs[23] controlling everything, automatically adjusting the lock-in amp’s sensitivity, translating the thermometry signals to temperature, saving the data files, and plotting the results. Relatively bug-free by the time we began our work on the copper oxides, this new system enabled us to perform much more accurate tests at a much faster pace.
Testing typically began on a new sample with simple four probe AC resistivity tests from room temperature down to 4 K. On occasion, magnetic susceptibility tests would be performed as well. An entry for each test was made in a hardbound laboratory notebook,[24] including the date, sample composition (sometimes abbreviated), type of test, data file name, inputs, and various notes.[25] All data was collected by the PC and saved to floppy disks. Incidentally, about fifteen years later, I challenged friends Drew Callan, Toby Flynn, and Matthieu Mollet to attempt to recover the data from the original disks. The file systems on many of the disks were damaged. Bit copies were performed, often with different results from each try. File fragments were often scrambled. The linked archive is my best effort at reconstructing the original results and can be cross-checked with any of my references to specific test results.[26]
By the following Monday, the 15th of December, we were testing our first copper oxides (UAH Lab Notebook page 11). While we initially recorded only “La-Sr-Cu-O” for the first several tests, my recollection was that the specific composition was La1.67Sr0.33CuO4 (as we will later note explicitly when we begin to vary the composition), one of the compounds studied by Michel and Raveau. With CaCO3 (the base mineral in limestone) even easier to come by than its strontium counterpart, La1.67Ca0.33CuO4 samples were prepared as well (UAH Lab Notebook page 12), guided in part by the earlier work of the French scientists as we worked up the alkali earth column in the periodic table (Ba, Sr, Ca, Mg).[27] Having run so many tests on “single digits Kelvin” superconductors, the results of the resistivity test on sample #SR1, for example, seemed unreal – the apparent onset of superconductivity around 35 K. A second test, #SR2, confirmed the results. The following day, a test on sample #CA1 (La1.67Ca0.33CuO4) showed its resistivity taking a downward turn around 15 K.[28]
Around mid-December, we received by mail some samples from the University of Houston. According to our records, at least one of the samples was identified in our notes as La3Ba3Cu6O14 (UAH Lab Notebook, page marked 12).

Some of the samples received from Houston by the UAH team.
I must pause here to point out that the La3Ba3Cu6O14 composition was almost certainly taken from a paper by the Michel-Raveau group; absent the more detailed structural analysis performed by the French scientists, Houston would have had little reason to normalize the composition to something other than La0.5Ba0.5CuO3-y (as indicative of the presumed perovskite-like structure), or possibly LaBaCu2O6-y if integer subscripts were preferred. This particular copy[29] came back to me by way of Ruling Meng (Chu’s lab manager) around 2009. Ironically, the Michel-Raveau group had, in the very same paper, explored partial substitutions of lanthanum with the other rare earth elements, including yttrium. In many ways, their compositions were even closer to the subsequently identified pure-phase YBCO superconductor than the initial mixed-phase material that will mark the discovery. Their paper further emphasizes the fact that the long-running (but irrelevant) contention about “who was the first among the Huntsville and Houston teams to suggest yttrium” is, at a minimum, no debate about brilliance on the part of anyone. It would seem to be more of a debate about who can read. While Chu would like many to believe that the Huntsville team’s work with yttrium was prompted by an idea of his, my recollection is that my “original” idea to at least consider yttrium was likely taken from this very paper, a publication whose existence prior to the discovery and its relevance to the copper oxide materials should be undisputed. I would additionally suggest that Chu’s “original” idea to consider yttrium likely came from the same source (as La3Ba3Cu6O14 almost certainly did). I will revisit the “whose idea was yttrium” question again later.

The first page taken from my old copy of the Er-Rakho et al. paper.
Whatever the nature of the Houston samples or the reasons they were sent to us (my recollections on this are uncertain), our tests showed only partial transitions around 30 K.[30] Four tests were conducted on two different pieces from the first batch. A fifth test was conducted on December 22nd on a piece from a second batch (UAH Lab Notebook page 13). Concurrent with these tests, we in Huntsville continued to process samples of La1.67Sr0.33CuO4, varying the processing temperatures and times as well as exploring slight variations in the lanthanum-to-strontium ratios (UAH Lab Notebook pages 13-14), with an eventual trend towards lower levels of alkali earth substitution for lanthanum.
Testing continued vigorously through the holidays with the exception of Christmas Day (see UAH Lab Notebook, page 14). It was some time during this period that I developed a concern about the inherent disorder of lanthanum and strontium in the so-called double perovskite materials. The base formula for most of our samples, La2-xSrxCuO4, could be thought of as a solid solution of La2CuO4 and Sr2CuO3.[31] In other words, lanthanum and strontium tend to randomly substitute for each other. Some discussions over Christmas with high-school friend Daniel Shultz,[32] who had just completed his ceramic engineering degree at Georgia Tech and recently enrolled as a graduate student at UAH, led to the idea of a makeshift ball-mill; if the physics was not conducive to a naturally ordered distribution, then ball-milling would at least help ensure that the starting mixture was at least as uniform as possible. My obsession with the La-Sr disorder will be strongly reflected in many of the compositions to be tested in January.
By the 27th of December, we had conducted a total of 29 resistivity tests and one magnetic susceptibility test on various La-Sr-Cu-O samples. Unfortunately, going into the latter part of the month, the pace of testing was evaporating our liquid helium at a faster-than-usual rate. With both the university purchasing office as well as government offices[33] closed for the holidays, we were unable to place an order for another tank.[34] As the last of our liquid helium evaporated, the minimum temperatures achieved in the last tests began to rise. With the school break, Wu decided that we should take a road trip to Houston to continue our testing. Chu will subsequently describe the trip as akin to going to receive our marching orders, something that could have been well-accomplished by phone. Liquid helium, on the other hand, is not so easily transferred by wire.
At the end of a 750-mile drive, testing resumed in Houston on the 29th of December (UAH Lab Notebook page 14) with emphasis, for the time being, on La-Sr-Cu-O samples under high pressures. I personally found the high pressure testing fruitless, but this had always been Chu’s interest and one that he had quite thoroughly imparted to Wu.
Early January 1987
Forty-two tests were conducted in Houston on test samples or lead (Pb) references through the early morning hours of January 3rd (UAH Lab Notebook pages 14-18). Later that evening, we headed back, staying overnight in a Motel 6 on I-10 in Louisiana before finally arriving in Huntsville on January 4th.
It would be January 9th before a new tank of liquid helium would arrive. A couple of days were spent processing existing data files and working with Daniel to make a crude ball mill for mixing and grinding samples. Concurrently, Daniel and fellow student Tony Xidis[35] worked with me to implement Daniel’s suggestion of using a coprecipitation method for fabricating the materials.[36] [37] Meanwhile, Wu’s focus shifted towards a more in-depth study of the existing materials, especially the La-Sr-Cu-O system. His efforts would require the very best samples, which I hoped would play well into my desire to produce more uniform materials. Since our last few tests in Houston on a sample with less strontium, namely La1.8Sr0.2CuO4, had produced some of our sharper transitions, our attention shifted to materials with less of the alkali earth substitution for lanthanum, and with the slightly altered composition, calcium was revisited in a La1.8Ca0.2CuO4 sample.
The first samples recorded after returning to Huntsville were milled versions of these two compositions, labeled “finely ground” in our notebook (UAH Lab Notebook page 18).[38] The tests on January 7th were impatiently tested in liquid nitrogen while still awaiting the arrival of liquid helium, as good metallic behavior at the higher temperatures would still be indicative of a better quality sample. Several of the calcium tests on January 9th and 10th (including CA7, CA8, and CA9) subsequent to the arrival of the helium showed complete transitions centered around 20 K, while a sample of La1.8Mg0.2CuO4, made about the same time, was found to be insulating.[39]
At this point, the stage was set. Working up the alkali earth column on the periodic table had produced the following results:
- La1.8Ba0.2CuO4, ~30 K superconductivity
- La1.8Sr0.2CuO4, ~40 K superconductivity
- La1.8Ca0.2CuO4, ~20 K superconductivity
- La1.8Mg0.2CuO4, insulating
A question then began to rattle around in my head concerning why the critical temperature peaked with strontium. It should be noted that many subsequent accounts out of Houston refer to frequent indications of “unstable” superconductivity as high as 70 K in samples containing barium. Thus, the barium compounds continued to be the focus of much of the Houston team’s work during this time. In contrast, we had seen no such indications, even in the Houston samples sent to us, and thus centered our attention on the strontium compounds, which showed the consistently highest transition temperatures.
My motivation for including these tedious details of the January activities is to counter many stories (several of which will be covered later in this narrative) that claim that the “direction” we supposedly received in Houston from Chu over the New Year’s holiday was to focus on yttrium materials. Together, Chu and Meng claim that the UAH strontium samples were of such “poor” quality[40] that Meng was tasked to take over the strontium work, but the UAH lab notebooks clearly show that the January focus continued to be on strontium compounds through at least the 23rd (UAH Lab Notebook page 24) while the Houston lab notes will indicate that a substantial part of their effort continued with barium. It will be years after the discovery when I first learn that it is the Houston team who makes the first yttrium samples and, with them, the first yttrium failures.
Following the 10 January results, the efforts over the next several weeks were intense, spawning several concurrent threads of activity. Thus, I must necessarily bounce around a bit as I try to weave in all of the relevant pieces and struggle to maintain a roughly chronological narrative.
With the second week of January, Wu began to spend increasing amounts of time at NASA’s Space Sciences Lab (about a six mile drive), especially in the labs of Frank Szofran (Hall effect) and Gretchen Perry (electron microscopy).[41] Based upon what records I could retrieve, Hall effect measurements were conducted on La-Sr-Cu-O samples[42] on January 7th, 8th, 12th, 13th, 19th, 20th, and 22nd.[43] Wu seemed to like the results obtained from the milled samples and used them for yet another series of high pressure measurements – 23 separate tests from January 11th through the 15th where my handwriting only briefly appears and then usually right before a test on a sample of interest to me (UAH Lab Notebook pages 20-22). Concurrent with both the Hall effect measurements and the latest series of high pressure measurements, Wu suggested that substitutions for the transition metal copper would represent a scientifically more significant finding,[44] thus beginning a series of La1.8Sr0.2CuO4 samples (UAH Lab Notebook pages 20-23) with the copper partially substituted with gallium (January 11th), aluminum (January 13th), silver (January 13th and 16th), and mercury (January 17th). To me, the choices seemed somewhat random, with emphasis often placed upon familiar elements. For example, much of our recent work had been with Ga-Bi and Al-In-Sn alloys.[45] Thus, gallium and aluminum topped the list. Silver shared the same column with copper, making it a marginally more logical choice (save its inability to form very stable oxides). A gold sample was similarly tried but stubbornly refused to oxidize, leaving visible gold particles within the matrix of the remaining material. The sample, which was deemed unworthy of testing, did strike me as sadly humorous at the time.
Third Week of January 1987
We were making poor decisions from ignorance, and clearly some education would be useful before pushing ahead. While I was a bit slow coming to this recognition, the more knowledgeable reader may have long since recognized that we were neophytes in the area of crystal chemistry.[46] Incidentally, I will shortly cover Chu’s references to atomic radii as somehow relevant to these ionic compounds,[47] an error that earns him a spot among the tyros as well.
During the prior fall quarter, I had taken my first solid state physics class, incidentally under Wu’s instruction. The text was Introduction of Solid State by Charles Kittel. Chapter 1 introduced elementary concepts of crystal structures, whereby atoms are modeled as simple hard spheres. Chapter 3 included a series of “periodic tables” with various properties of the elements – cohesive energies, melting points, bulk moduli, compressibilities, ionization energies, and then several pages later, atomic and ionic radii. It was this text to which I turned upon examining the curious trend in critical temperatures as the alkali earth elements were replaced in sequence. It seemed reasonable to me by mid-January to seek some property of those elements that demonstrated an extreme value with strontium. Unfortunately, the trends all appeared monotonic or irregular. With the last of the tables, though, I did notice something curious – from among the alkali earth metals, the ionic radius[48] of strontium (1.13 Angstroms) most closely matched that of lanthanum (1.15 Angstroms), for which it substituted. It seemed likely that the reason strontium samples produced the consistently highest critical temperatures was because, as it replaced the only slightly larger lanthanum, the degree to which it perturbed the crystal lattice was minimized. In short, it was the best fit.[49]
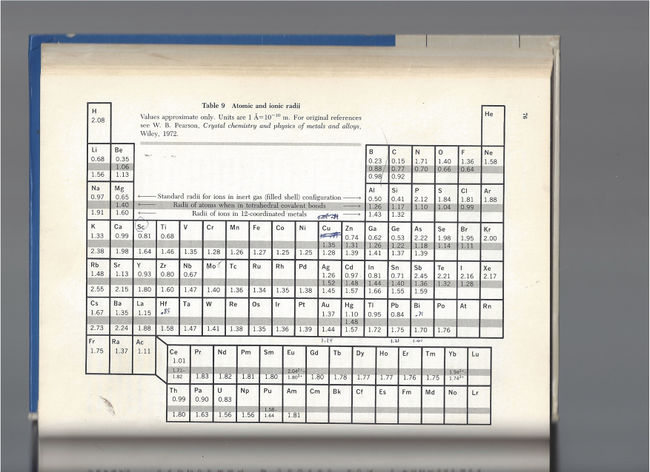
Copy of the “Atomic and ionic radii” table from my textbook Charles Kittel. Introduction to Solid State Physics, Sixth Edition. New York: Wiley, 1986.
I think it a good place here to address a couple of issues regarding the various “histories” of the discovery. It will be demonstrated soon that my ideas will take on a very important role in directing the compositions examined in Huntsville, and for a brief period immediately after the YBCO discovery, these ideas will even direct the compositions made in Houston. The final piece of evidence in support of this claim will actually come from the Houston lab notebooks, and with great precision. Of course, Chu’s claimed approach was fundamentally very different from mine. His numerous patent applications during the course of 1987 (beginning with his 12 January filing[50]) discuss at great lengths the idea of simulating the effect of pressure by “reducing interatomic distances” through substitutions of smaller atoms.[51] Chu never references the more applicable ionic radii (at least through the spring of 1987),[52] instead noting the largely irrelevant (although roughly correlated) atomic radii of the elements of interest.[53] He lists the atomic radius of barium as 2.22 Angstroms, strontium as 2.15 Angstroms, calcium as 1.97 Angstroms, and lanthanum as 1.87 Angstroms. Thus, he was unlikely to have ever made the matching-size observation I did given that with these values calcium, not strontium, is the better match to lanthanum. In any case, his argument seems to always be that smaller must be inherently better (as supposedly guided by his high pressure observations). Finally, given the very fundamental importance of atomic/ionic size in crystal chemistry, the idea that the Huntsville and Houston groups were independently giving these kinds of factors consideration should not be difficult to accept, especially since the specifics of the application were still very different.
Over the course of the subsequent patent dispute, endless pages were devoted to debating the question of who among the Huntsville and Houston teams was the first to utter “yttrium.” I direct the reader’s attention to the list of “set-the-stage” compositions above as well as the table in the excerpts from Kittel’s textbook.[54] At this point in the story, it was known that combinations of lanthanum with strontium and, to a lesser degree, lanthanum with barium produced the consistently highest critical temperatures in the copper oxides. Note in the table from Kittel that lanthanum, barium, and strontium form four quadrants of a larger rectangle with yttrium being the fourth quadrant. Thus, I would suggest that the ridiculous dispute over who was the first to mouth “yttrium” is the functional equivalent of the same individuals, having taken a hypothetical group trip to the Four Corners Monument, foolishly arguing over who first had the idea of stepping on Colorado. Lastly, I must emphasize that ideas for new compositions were being thrown out at a brisk pace by virtually everyone in both labs, a pace far faster than samples could possibly be made or tested, and, as will be shown when I cover the Houston patent applications in greater detail, no one could best Paul Chu at throwing out compositions at a blistering rate.
Returning to the core narrative, with the repeatedly disappointing results for the copper substitutions, combined with my now increased sensitivity to ionic radius, I waded in towards the end of Wu’s copper substitution series with one sample of my own to verify my suspicions that copper replacement would be an especially difficult path to higher critical temperatures. More carefully syncing valence and size to the copper, I had selected zinc (a firmly divalent choice with an ionic radius of 0.74 Angstroms very closely matching that of divalent copper). Fully expecting the partial substitution with zinc to destroy the superconductivity, the test on the 17th of January (UAH notebook page 23) confirmed my anticipation. The zinc, in fact, completely eliminated all metallic behavior, yielding a semiconducting resistivity curve trending ever upward with decreasing temperature. This sample would mark the final attempt at copper alternatives and the motivation for another trip to the library.
With evidence mounting for the importance of carefully selecting elements with the appropriate valence and ionic radius, I refocused my attention on substitutions of the other metals. My dissertation, defended in November of 1990, describes the next steps:
Of course, the first step was to try substituting stable divalent cations of the appropriate size in order to test the postulate. Because partial replacements of copper in (La,Sr)2CuO4-y with various nonmagnetic ions had already shown mercury to be among the least detrimental to the superconductivity (17 January), it was assumed that the relatively large Hg2+ ion (radius 1.10 Å) was actually occupying the lanthanum and strontium sites. However, given the low decomposition temperature of mercuric oxide (500 °C) and the extremely high vapor pressure of the metal above that temperature, the possibility that no mercury would remain in the samples after reaction was acknowledged. Nevertheless, considering its ionic size and stable divalent state, it was reluctantly chosen as the only element that could potentially rival strontium.[55]
It should be noted that from the ionic radius table from Kittel, mercury would seem to easily represent the best combination of valence and size to strontium. The actual test results on the ensuing mercury sample and their important role in the discovery will be revisited when I come to that point in the timeline.
The La1.8Ae0.2CuO4 (Ae=an alkali earth element) samples that had been the focus of our work were known somewhat interchangeably at that time as the “K2NiF4” or “double-perovskite” phases, in reference to their crystal structure. This provided me one key word, “perovskite,” as the basis for my library search. The first text of note that I found was entitled Structure, Properties, and Preparation of Perovskite-type Compounds.[56] Since naturally-occurring minerals with the perovskite structure are relatively common, I was also inevitably directed towards books on mineralogy. One in particular bore the title Mineralogy: Concepts, Descriptions, Determinations, and just inside its front cover was a simple chart of ionic radius vs. ionic charge for various elements (and furthermore showing La3+ and Sr2+ as having the same radius – probably part of the reason the table caught my eye).
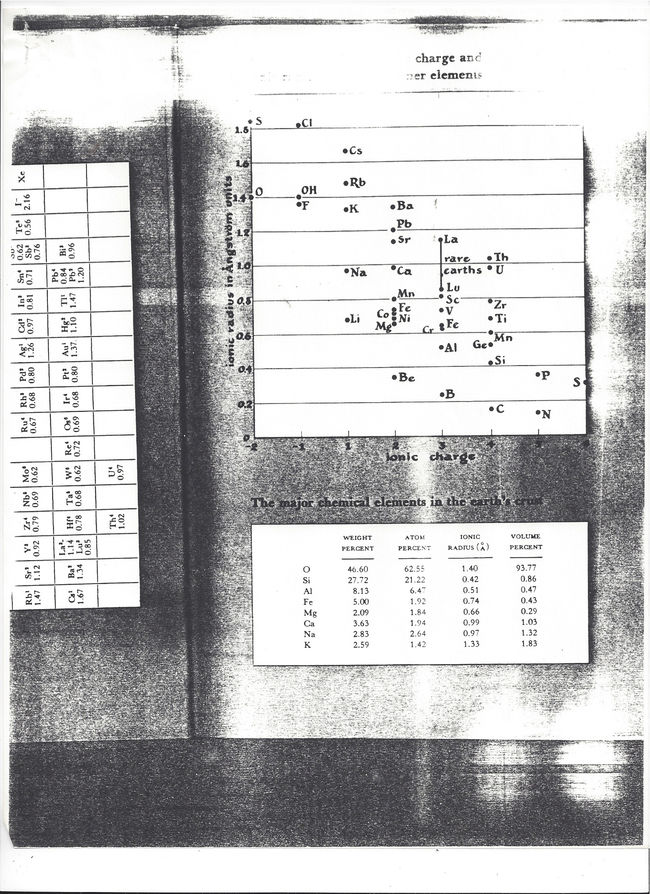
Copy of the ionic radius chart from L. G. Berry, B. Mason. Mineralogy: Concepts, Descriptions, Determinations. San Francisco/London: W. H. Freeman and Company, 1959.
Shortly thereafter I created my own hand-drawn version that became a convenient tool in the formulation of candidate superconductors.[57] Galasso’s perovskite book, however, was easily the most critical piece of the puzzle, in particular this line:
The largest group of complex perovskite type compounds has the general formula A(B’0.5B”0.5)O3. When the structure of the compounds is ordered, and most of them are, they adopt the structure shown in Fig. 2.5. It was postulated by Galasso et al. that an ordered distribution of the B ions is most probable when a large difference existed in either their charges or ionic radii.[58]
The statement refers to the smaller “B-site” in the crystal structure, but it seemed reasonable that the same principle could be easily applied to the larger “A-site.” With this, I seemed to have found a solution to the problem of the alkali earth substituting for lanthanum in a disordered manner – instead of trying to match lanthanum’s size perfectly, force an ordered distribution by replacing lanthanum and/or strontium with suitable substitutes having a larger size difference, thereby coercing an ordered distribution. Galasso’s book described how, to preserve the packing critical to preserving the basic structure, the relative radii of the metal ions had to fall within certain constraints, or, in the case of a mix of metals in a given site in the crystal lattice, the weighted average of the radius had to fall within a particular range. My dissertation describes my conclusions this way:
Two constraints on the selection were assumed necessary. First, the average valence of the A ions should remain between 2.5+ and 3+ and preferably close to 2.9+ as with (Lao.9Sr0.1)2CuO4-y in order to preserve the mixed valency of the copper essential for conductivity. Second, in light of the extreme sensitivity of perovskites to the ionic sizes of their component ions, the weighted average volume of the A ions (or simply radius cubed, <r3> = 1.51 Å3 in the case of the A’ = Sr2+, x = 0.1 compound) should be preserved in order to support and preserve the structure. The arguments for average volume as the appropriate constraint will be given elsewhere.[59]
Given the passage of twenty-five years, it is perhaps time to finally explain why average volume was chosen over average radius.[60] Beginning by noting the quasi-two-dimensional nature of the La1.8Ae0.2CuO4 (Ae=an alkali earth element) materials, I envisioned a single CuO2 plane from the structure and equal amounts of two different large metal ions (i.e., A’1.0A’’1.0CuO2). Now, instead of layers of A ions above and below the plane as in the actual structure, I simplified the problem to two dimensions by dropping one set into the plane.
My original derivation was lost, but I have included below a reconstruction done in the course of the YBCO patent battle. The circles along the edges represent oxygen ions, whose radius I neglected (in hindsight, a serious omission given that they are relatively large). The smallest circles on the vertices represent copper. Assuming the copper-to-oxygen bond length to be a constant length represented by the script letter “l,” one can derive a relationship suggestion that the sum of the squares of the radii of A’ and A” be held constant. I then simply presumed this result to extrapolate to a weighted sum in the event the relative amounts of A’ and A” differ from one-to-one and then to weighted volumes in the case of a three-dimensional problem. It should be noted here that as Wu was only vaguely familiar with the details of my efforts, it is at least remotely possible that my attempt at maintaining a constant copper-oxygen bond length overlaid with Chu’s subsequent attribution of the discovery to insights gained from high pressure measurements led Wu to morph Chu’s “reduced interatomic spacing” into the compromise term “optimal interatomic distance,”[61] thus crudely capturing the essence of both reality and Chu’s fantasy in a single phrase.[62]
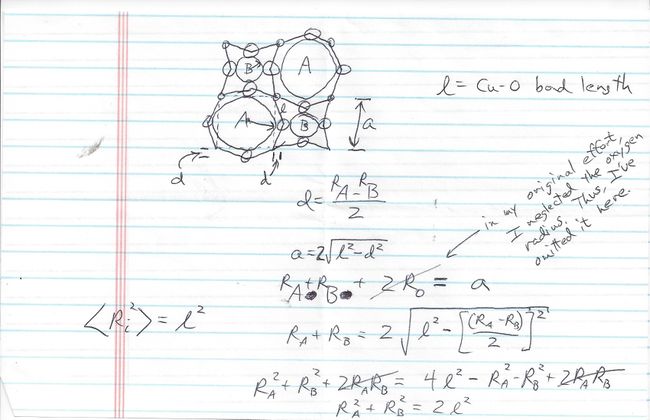
Reconstruction of derivation towards preservation of Cu-O bond length.
My dissertation again describes the next steps. I apologize for the lengthy quote but it would be difficult for me to describe the process now any better than when I first drafted this so many years ago:
Since the sources consulted implied that even the largest quadravalent cations (e.g., Ti4+, Zr4+, and Pb4+) exclusively occupy the smaller B site in perovskites (as in the former high-Tc record holder for perovskites – Ba(Pb,Bi)O3) and later tests on January 18 and 19 suggested that the monovalent alkali metal ions Na+ and K+ (with sizes similar to Ca2+ and Ba2+, respectively) could not successfully substitute for strontium in (La,Sr)2CuO4-y, the possibility of increasing the difference in valence was eventually ruled out. Restricted to divalent and trivalent cations, all of the constraints could not be met concurrently. Since preservation of the structure was given highest priority, the precedence was assigned beginning with average volume, followed by average valence, and finally a near one-to-one ratio.
Because La3+ is the largest stable trivalent cation, a combination of a smaller trivalent cation and a larger divalent cation was the only alternative. Since partial substitutions of copper with the smaller nonmagnetic ions of Ga3+ (11 January) and Al3+ (13 January) had already been found to suppress superconductivity in the (La,Sr)2CuO4‑y compound, the size of the Cu3+ ion was assumed to represent an extreme lower bound on the size of the A ions. Thus, La3+ yielded to the smaller Y3+ ion (radius 0.93 Å) while the large Ba2+ ion (radius 1.35 Å) regained its position over Sr2+. Other possible trivalent cations were considered less promising because of unstable or high vapor pressure oxide forms, unpredictable valence states, large magnetic moments, and so forth, and some have since been proven suitable, or even superior, substitutes for yttrium, including thallium, bismuth, and most of the rare earth elements. The uncommon occurrence of perovskites with Y3+ as the exclusive A ion suggested that materials too close to the composition Y2CuO4 were unlikely to be isostructural with the La2CuO4 compound. Binary oxides containing yttrium and a smaller metal ion tend to prefer structures such as spinels or garnets over perovskite or perovskite-like structures.
Restricted to an average ionic volume of the A ions dictated by (La0.9Sr0.1)2CuO4‑y and adjusting x in (Y1-xBax)2CuO4-y to match it, the composition (Y0.575Ba0.425)2CuO4-y was calculated and found to have a low but tolerable average valence of 2.575+ and a near one-to-one ratio of yttrium and barium. Since the composition approached the limit of the ability of the anticipated structure to maintain its stoichiometry through the formation of oxygen vacancies and trivalent copper and an ordered distribution of yttrium and barium ions would necessarily incorporate them in a ratio of small integers, the composition was later, and rather subjectively, rounded to the 3:2 ratio of (Y0.6Ba0.4)2CuO4-y. Little consideration was given to the possible exact nature of the ordering and its implications for the crystal symmetry.[63]
Thus, some time on the weekend of the 17th and 18th of January, the composition (Y0.575Ba0.425)2CuO4, or equivalently Y1.15Ba0.85CuO4, appeared in my undated scratch notes alongside La1.8Hg0.2CuO4. That weekend proved to be an especially convenient time for me as, being one week before the Super Bowl, it represented the first weekend of the year without a meaningful [American] football game (the Senior bowl does not count).[64]

My undated scratch notes from the weekend of 17-18 January 1987.
It is perhaps a good time to use my scratch notes here to point out that the oxygen ratio was frequently omitted in our shorthand for the candidate compositions. Technically, the chemistry convention is that an omitted subscript implies a one, but in the case of these mixed-valence materials, the precise oxygen concentration in the processed samples was an unknown, a function of the processing conditions, and irrelevant to weighing out appropriate amounts of the starting materials (which could be oxides, carbonates, or even hydroxides of the appropriate metals). (Unfortunately, the patent court would ultimately interpret the frequently-omitted subscripts as implied ones and, ignoring a decade of arguments made by both sides in the case, decide against UAH based upon a point never raised by either party – that we made, quite impossibly via heating in air or pure oxygen, some kind of bizarre (Y0.6Ba0.4)2CuO1 “sub-oxide.”[65] [66])
Up to this point in our work with the copper oxides, we had been able to locate every reagent of interest from somewhere within the UAH Science Building, usually the stockrooms that supplied the teaching labs. My pocket calendar will note on January 9th, for example, “Check P. Chem. & Guy Smith’s labs.”[67] [68] Unfortunately, yttrium proved not so easily located. At this point, I consulted friend Daniel Shultz again. One must know Daniel to fully appreciate his energetic and contagious enthusiasm, but with my inquiry about where to best locate yttrium, his ceramic engineering background activated, and he began a lengthy and excited description of the amazing properties of yttria-stabilized zirconia, finally culminating with the suggestion that a ceramist would almost certainly possess some yttrium oxide. I knew of only one, Ed Ethridge at NASA’s Space Sciences Lab, the same building to which Wu was still making frequent trips in support of his more detailed studies of (La0.9Sr0.1)2CuO4. Friend and fellow student Jones Hamilton,[69] who frequented our lab to follow the latest developments, waded in about that time. I shared with him some of the cursory details of my ideas which, when combined with his independent fascination with the element yttrium, led him to quickly became the element’s biggest advocate.[70] Some years later, I asked him to compile his recollections, which he did and subsequently provided in a handwritten letter.[71]
Consistent with Jones’ recollection, I asked Wu at least twice before his trips to NASA’s Space Sciences Lab to try to get some yttrium oxide from Ed Ethridge. On perhaps the second trip (probably on or about the 23rd of January), he returned with a supply of 99.99% pure yttrium oxide bagged inside a quart size “paint can” that appeared to have never been opened.
I very recently contacted Ed Ethridge via LinkedIn to ask him of his recollections. Here is his response:

LinkedIn Message from Ed Ethridge to Jim Ashburn, 29 July 2015.
Combining Ethridge’s specific recollection of a Thursday with my estimate of January 23rd allows me to refine the date the yttrium oxide was acquired to Thursday, January 22nd. As will be shown shortly, the rest of his timeline is early by one week, but his days of the week (for processing, testing, the trip to Houston) are relatively accurate. In any case, he notes a very short timeline between the request for yttrium oxide and the discovery, inconsistent with Robert Hazen’s account (to be covered in detail later) and difficult to reconcile with the Houston story of Huntsville being tagged with the “yttrium task” in early January because we would be able to quickly obtain yttrium oxide from NASA.
Having recently followed up with Ethridge in the wake of the above message, I found that he had, in fact, located the old yttrium oxide container. We arranged to meet at Wilson Hall (the former UAH Science Building) where I posed for a picture with the can in exchange for a close-up of it.

Can of Yttrium Oxide borrowed from Ed Ethridge, NASA Space Sciences Lab.
Given the story the Houston team will (in the course of my narrative) put forth to the USPTO of a YBCO discovery in Houston independent of the Huntsville team, it seems odd that Ethridge would have kept a reagent container for over 29 years now if he did not have every reason to believe it significant (reasons probably firmly entrenched in his mind well before the various myths and legends began to take root), but that analysis will be an exercise ultimately left to the reader. If there is any doubt concerning the age of the can, I would note that Consolidated Astronautics, by my best estimation, ceased to exist (at least under that name) around 1993.
Jumping ahead briefly to 2006, Ruling Meng (in her “perjury” affidavit – linked later when I cover it in detail) will claim to have been the one who suggested “NASA” as a source for yttrium oxide during our New Year’s trip to Houston. One can only imagine how she knew that upon our return to Huntsville we would be unable to locate yttrium oxide in the various stockrooms and labs we routinely checked within the UAH Science Building, places where we had successfully, up to that point, found every other reagent we had sought. Furthermore, suggesting we try “NASA” would not be particularly helpful given that, at least as of April 2008, Marshall Space Flight Center had 7000 government employees and 170 buildings on about 1800 acres (presumably all of these numbers were substantially higher during the peak years of the Space Shuttle program). I have recently been inquiring of several NASA personnel about the possible existence of a chemistry stockroom at the Marshall Space Flight Center. So far, I have found no one with any knowledge that there is or ever was a dedicated chemistry stockroom or its functional equivalent at the center.
It seems far more plausible to me that Ruling later read of the yttrium oxide search in the May 1987 U. S. News & World Report article[72] and subsequently convinced herself that she had prompted Wu. Not coincidentally, that article, in the same paragraph, mentions both the yttrium oxide coming from NASA and happens to quote me (I was rarely interviewed for national publications as those reporters typically contacted only Houston). I do not specifically recall speaking with that particular author, but I have no doubt that I was the one who related the story of searching for and obtaining the yttrium oxide. It was a significant event in my mind because so many friends assisted my search.
Incidentally, just prior to the above, the article provides a bit of levity in describing the supposed key moment that yttrium was targeted (my emphases):
Early in January, he [Chu] and his partners hunkered down over old data and worked their way through the periodic table. Finally they got to yttrium [aka Colorado].
In the course of my prior discussions with Daniel on yttrium oxide, he pressed upon me some information that gave me pause in cracking the newly-received can by describing the critical importance to a ceramist of preserving the purity of his chemicals. I had never before worked with a material of such high purity and was quite honestly fearful of contaminating it. Thus, the can of yttrium oxide sat on the shelf for about five days as I turned my attention to more samples made with the student-grade materials, namely (La0.9Hg0.1)2CuO4.
Through the month of January, the different handwriting in the lab notebook offers a good indication of the relative interest between Wu and myself in what had become, at least for now, mostly parallel efforts. By Monday, January 19th, Wu’s handwriting[73] had all but disappeared from our notebook, appearing only once on January 23rd (UAH Lab Notebook page 24) with a test marked “Hall Effect Sample” that even includes a sketch of the leads attached in the arrangement used for such testing.
Late in the writing of this narrative, I tracked down an email (dated 23 November 2011) that I had received from Daniel having asked him to document any recollections he had from the weeks leading up to the YBCO discovery. While the email does spoil a bit of the story, this point otherwise seems as good as any to interject it.

Email from Daniel Shultz recounting his memories of the events leading up to the YBCO Discovery.
There are several items of note. First, Daniel describes our coprecipitation and ball-milling work to “insure stoichiometric mixing of the components” and that those efforts extended into January. Second, he mentions our discussions about crystal structures and what he describes as “impurity effects,” in this context referring to the alkali earths that substitute in the La2-xAexCuO4-y materials. Third, he mentions my “hand-drawn table of element sizes and volumetric proportions,” referring to my chart of ionic radii and my corresponding “volumetric” considerations, and that the table had led me to believe that I could actually make yttrium work. Finally, it is a bit interesting that there is no mention of Wu (much less Chu) in his email. Granted, Daniel, by virtue of our longtime friendship, is not likely to be counted among the least biased of witnesses, so I will simply leave it to the reader to assess his credibility.[74]
Final Week of January 1987
I took a break Super Bowl weekend, and if Wu came into the lab over those days, he apparently did no resistivity or susceptibility testing. By the following Monday, the mercury sample was ready for testing and became a pivotal event in the moments leading up to the YBCO discovery. Again, from my dissertation:
Yttrium oxide was finally obtained on Friday, 23 January just as the first (La1‑xHgx)2CuO4-y sample was being made. After 24 hours at 995 °C in air, it was removed from the furnace and tested the following Monday with some encouraging results – a stable, reproducible resistance drop around 38 K (just as anticipated based upon the similar ionic sizes of Hg2+ and Sr2+), apparent evidence that at least some mercury had indeed been retained in the material. From 26 to 28 January, transitions were detected in all seven tests on four different samples (two with x=0.075 and two with x=0.125) with onsets ranging from 37.0 to 39.5 K. The importance of ionic size and its connection with Tc seemed certain.
January 26, 1987 test results on La1.85Hg0.15CuO4.
The transitions in the mercury samples were partial, but the temperatures were precisely as expected.[75] My dissertation continues with the story:
…on 28 January, motivated by the latest [mercury] results, yttrium oxide, barium carbonate, and copper oxide were mixed in the appropriate amounts to make (Y0.6Ba0.4)2CuO4-y, thoroughly ground in a mortar and pestle, pressed into pellets, and placed in a furnace in air at 996±2°C for 24 hours, the same conditions previously used for most of the (La,A’)2CuO4-y materials.[76]
The tests on the mercury materials seem to have gotten Wu’s attention, but, as was his nature, he preferred a more systematic approach. Thus, when the container of yttrium oxide was finally opened, he asked that I make a sample of Y1.8Sr0.2CuO4, a straight substitution of lanthanum with yttrium in the then consistently highest-Tc superconductor La1.8Sr0.2CuO4. I did not express it at the time, but there were two reasons I was opposed to even trying Wu’s composition. First, I knew that there would be too many small ions to support a crystal structure even remotely like that of the (La,A’)2CuO4 materials. Second, other groups would have almost certainly tried such a straight substitution anyway.[77] Incidentally, the YBCO samples that will mark the discovery have long since disappeared (despite my many efforts to locate them[78]), but I do happen to still possess the container with Wu’s failed Y1.8Sr0.2CuO4 material.

Y1.8Sr0.2CuO4 sample container from UAH lab.
At the last second, I chose to slightly alter my original YBCO formulation. My dissertation states, “Since… an ordered distribution of yttrium and barium ions would necessarily incorporate them in a ratio of small integers, the composition was later, and rather subjectively, rounded to the 3:2 ratio of (Y0.6Ba0.4)2CuO4 [equivalently, Y1.2Ba0.8CuO4].” That precise composition, which would very soon become the basis for the paper announcing superconductivity in YBCO, appears in this copy of my scratch notes.
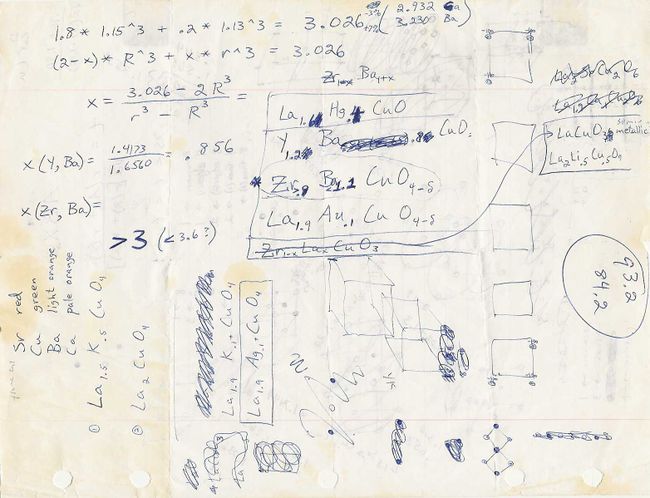
My scratch notes page (undated) showing the first appearance of the Y1.2Ba0.8CuO4 composition.
While I made Wu’s Y1.8Sr0.2CuO4 sample, I asked C. J. to make my Y1.2Ba0.8CuO4 sample. Both went into the furnace together. The next day, C. J. called in sick (and was unfortunately not present for the discovery). Upon removal from the furnace, Wu’s Y1.8Sr0.2CuO4 sample was found to be a favorable black-to-dark-gray color. A quick test with an ohmmeter showed it to be insulating. Thus, it was not tested. The Y1.2Ba0.8CuO4 sample, on the other hand, was a discouraging greenish color. My dissertation describes the events that followed this way:
…the pellets [of YBCO] were… found to consist of black particles embedded in a green matrix. Despite the overall greenish color, they were not insulating. A small bar was cut, fitted with platinum leads, and lowered into a liquid helium dewar for testing… Just below 90 K, the resistivity began to drop rapidly, reaching zero (i.e., below the detectability threshold of the apparatus) near 50 K.
The results of that first test are shown here.
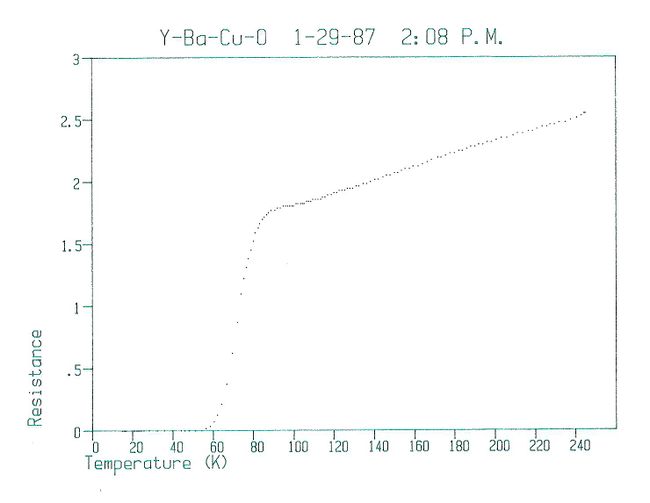
Results from first test on Y1.2Ba0.8CuO4 from the afternoon of January 29, 1987.
My dissertation continues:
Fearing the possibility of false temperature readings, the same piece was quickly retested in liquid nitrogen, again with an onset near 90 K. As another batch went into the furnace, a second piece from the original batch was tested with similar results. Tests on samples from the second and third batches, impatiently removed from the furnace after two and four hours, respectively, were found to have even higher and sharper transitions. In all, eight tests on four samples from three separate batches were performed that day. All showed transitions with onsets ranging upwards from 89 K and averaging 93 K and midpoints nearing 93 K.
The resistivity of sample #3 (one of the better samples) is shown here.
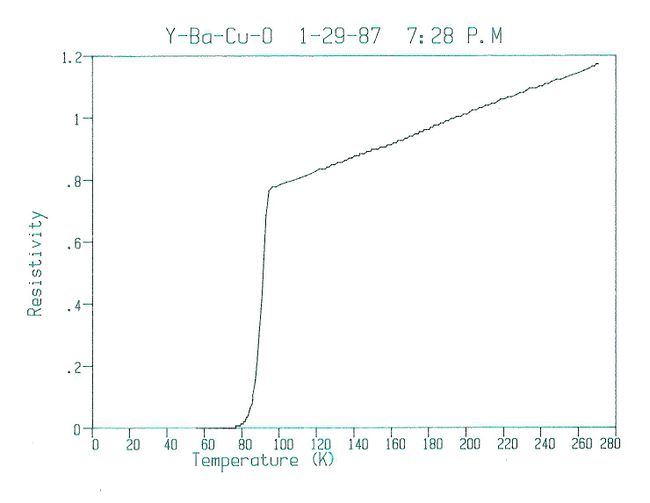
Some of the better results from January 29, 1987.
Some time over the course of the afternoon, two other events of note occurred. I cannot speak with certainty to the order in which they occurred.
First (not necessarily chronologically), Wu contacted Houston to tell Chu what we had observed. Chu will later record that the call occurred sometime around 5 p.m., and I would suggest that this is probably correct. It is my understanding that Wu first spoke with Pei-Herng Hor (one of Chu’s graduate students), but in addition to my hearing only one-half of the conversation, it was mostly in Chinese.[79] Sometime later that evening, perhaps after a second phone call with Chu, Wu decided we would go to Houston for the more definitive magnetic measurements.
Note that Wu was convinced that without those measurements, it would be impossible (especially for UAH) to push a paper through a sufficiently reputable journal. I also recall during that general period expressions of concern (real or imagined, and by specifically whom, I do not recall) about referees holding up papers and leaking information to “competitors.”[80] [81] Incidentally, Chu, who would become the de facto spokesperson for the effort, would demand and be granted a single referee for the paper. I will later discuss additional measures he would take to protect the details of the YBCO composition.
Second (again, not necessarily chronologically), Wu met with the chairman of the UAH Physics Department, Graeme Duthie.[82] I only learned of the details later through Duthie, who stated that, upon learning of the very loose and informal relationship between the Huntsville and Houston groups, he had urged that Wu and I go to the University of Oslo to perform the additional measurements. I can only presume that Duthie had friends there. Whatever the case, Wu decided that his former advisor Chu was the person most willing and best able to protect us from the mystical processes by which scientists so often lose credit for their discoveries.
On the morning of January 30th, samples in hand, we boarded a plane for Houston. Incidentally, we took the borrowed yttrium oxide (assuming Houston to have none; after all, we had no more knowledge of what they had been making than they of us), and I specifically recall being a bit nervous about the idea of having to explain to airport security why I was carrying a tightly sealed container of hundreds of grams of fine white powder.
The reader may note in the UAH lab notebooks that the tests on 29 January record the composition Yb1.2Ba0.8CuOy instead of Y1.2Ba0.8CuOy. “Yb” represents the element ytterbium, not yttrium. This regrettable move occurred because shortly after the press conference that would take place in Huntsville some weeks later I caught one of the reporters nosing through our lab notebook. I panicked (given that the composition was being kept secret until the paper was published) and inserted a “b” into the conveniently large space near the heavily slanting “Y.” I never imagined at that point that a patent interference would ensue between Houston and UAH and that the lab notebook would become necessary evidence. At the time, I naively believed that once the paper was published with the Huntsville team listed first, the claim would be secure. While the change in the Huntsville notebook was entirely my (very foolish) decision, a “Yb-for-Y” alteration was decidedly not my original idea. I will simply offer the documentation here in support of that assertion, direct attention to the relevant parts, and leave it for interpretation.
The first item is a page from Ruling Meng’s notebook.[83]

First page from Meng’s Exhibit H as submitted in the patent interference.
Note first the date of “29-30 January 1987” on the leading page. The correct date for this page is 30 January 1987, the date of my arrival with Wu in Houston; the “29-” is a later “edit,” as Meng does not routinely allow space after the day number to insert something akin to “-30” in the event the activities of the page span multiple days. In any case, Wu and I will be present in Houston at the time the contents of this page are penned. Now note the last composition on the first page and the first composition on the second page. A small “b” has been clearly inserted next to the Y, even though the metals and raw material weights indicate yttrium. This narrative will return shortly to these pages as they also include the most compelling evidence in support of my account of the YBCO discovery.
The second item is a galley proof retrieved from microfilm at least twelve years after the discovery.

Galley proof of originally submitted version of the joint UAH/UH paper. Incidentally, Chu was the corresponding author. From Physical Review Letters.
Note that three times (title, abstract, and body), “Yb” occurs where subsequently “Y” will appear in the final published version. I have very specific recollections of how this series of “typographical errors” came to be, but I will defer to Science Magazine’s Gina Kolata and her smartly entitled article to explore the potential causes.[84] Concerning the “effects” of such “typographical errors,” I have included here several copies of preprint requests received at UAH over the subsequent weeks.

Preprint requests received by UAH during the weeks subsequent to the discovery.
The reader who is somewhat familiar with the more prominent versions of this story that have appeared over the years may conclude that my story, being so very different, could simply be a clever fabrication built largely upon an “average ionic volume” coincidence, discovered after the fact, between La1.8Sr0.2CuO4 and Y1.2Ba0.8CuO4. I will now address those potential concerns using, ironically, the same pages from Meng’s notebook above.
Upon our arrival in Houston, Wu and I described my ideas to Chu, showing him my ionic radius chart and explaining how I had selected a pair of metals whose ions were both larger (barium) and smaller (yttrium) than the lanthanum/strontium tandem and in proportions that would fill the same space in such a way that it might preserve the distance between the copper and oxygen atoms. He was apparently sufficiently intrigued that I was asked to attempt to formulate additional new compositions based upon my premises. Unfortunately, Y1.2Ba0.8CuO4 had been the last of my more promising ideas. Nevertheless, I made an effort (hardly my best), the results of which just so happen to appear in Ruling Meng’s lab notebook.
Returning to the page above from Meng’s Exhibit H that displayed the “Yb error” reveals a series of compositions with curiously precise (three digits) ratios. Five appear on that page with the pattern:
- [La1-x(YyBa1-y)x]2CuO4
I will be focusing on the first three; the fourth and fifth listed simply vary “x” without updating “y.” The raw material weights appear on the bottom of that page through the next two pages. The page stamped H53 includes one additional composition of interest (numbered “6”) following the formula:
- [La1-x(LuyBa1-y)x]2CuO4.
Again, additional compositions are listed that simply vary “x” without updating “y.”
The formulas reflect the hastily cobbled idea of substituting for strontium a pair of larger and smaller ions in (La0.9Sr0.1)2CuO4, the composition that had been the basis for the original (Y0.6Ba0.4)2CuO4 formula. An almost identical idea was manifest earlier in samples of La1.8(Ba0.5Ca0.5)0.2CuO4 and La1.8(Na0.6K0.4)0.2CuO4 that I had tested on January 18th in Huntsville.[85]
The mapping of corresponding compositions by sample number is as follows:
- YB-104 → LYB-1 → LLB-1
- YB-105 → LYB-2 → *
- YB-103 → LYB-3 → *
- YB-102 → * → *
- YB-101 → * → *
The asterisks represent samples that, for whatever reasons, were never made. The corresponding compositions are:
- (Y0.2Ba0.8)2CuO4→[La0.9(Y0.155Ba0.845)0.1]2CuO4→[La0.9(Lu0.149Ba0.851]2CuO4
- (Y0.3Ba0.7)2CuO4→[La0.9(Y0.26Ba0.74)0.1]2CuO4→ *
- (Y0.4Ba0.6)2CuO4→[La0.9(Y0.378Ba0.622)0.1]2CuO4→ *
- (Y0.6Ba0.4)2CuO4→ * → *
- (Y0.8Ba0.2)2CuO4→ * → *
Prior to the appearance of the four rightmost compositions (and not coincidentally, prior to our arrival in Houston), non-integers appearing in the sample formulas in Houston generally come in pairs such that the fractional substitution of the divalent element (most commonly barium or strontium) for the trivalent element (typically lanthanum) is usually drawn from the set of simple decimal fractions {0.5, 0.4, 0.3, 0.2, 0.15, 0.1, 0.05, or 0.025} and, in any case, is never specified to more than two significant digits.
Thus, numbers like 0.155, 0.845, 0.26, 0.74, 0.378, 0.622, 0.149, and 0.851 are completely outside of the patterns that preceded them. Not coincidentally, upon our departure from Houston late on January 31st, the earlier rules quickly resume.
The mapping process for the above compositions is relatively simple and is basically identical to what I outlined in my dissertation – simply adjust the relative amounts of the non-copper metals until their average ionic volume is preserved. Doing the yttrium case first, the equations are as follows (canceling the 4/3 π factor from the sphere volume formula):
- x RY3 + (1-x) RBa3 = 0.9 RLa3 + 0.1 [y RY3 + (1-y) RBa3].
Before proceeding with the derivation, I will point out a couple of symbols and numbers that appeared along the margin of one of my scratch notes pages:[86]
- Y .8044
- Ba 2.4604
While the symbols are cut off, the numbers are clearly visible in the copy of this page as it appeared in the August 1988 Science article entitled “Superconductor Credits Bypass Alabama.”[87] Thus, these numbers were in place at least two years before the first copies of Houston lab notebook become available for review by the Huntsville party in the course of the ensuing patent interference. If one looks up the ionic radii values for yttrium and barium from Kittel’s aforementioned table (numbers noted previously in my dissertation excerpts) and cubes those numbers, the results are:
- Yttrium: 0.804357
- Barium: 2.460375.
These figures, when rounded to four digits right of the decimal, perfectly match those of my notes. Similarly, my other linked scratch notes page[88] includes “weighted volume” formulas down the right margin as part of what is much of the derivation of the YBCO formula. A copy of this page appeared in the Winter 1988 edition of UAH Magazine,[89] again well before copies of the Houston lab notes became available to the Huntsville party. A reconstruction of the equations as they appear is as follows. To match the ionic volume of the Y/Ba combination in Y2-xBaxCuO4 to that of the La/Sr combination in La1.8Sr0.2CuO4 (as described in my dissertation), the following quantities must be equated (again, dropping the 4/3 π):
- 1.8 RLa3 + 0.2 RSr3 and
- ( 2 – x ) RY3 + x RBa3
A normalized version of this equality will appear in the continued derivation of the “Meng numbers” below. Plugging into the first expression the ionic radii from Kittel yields the first equation appearing in my scratch notes,
- 1.8 * 1.153 + 0.2 * 1.133 = 3.026
Setting the second quantity equal to this result yields the second equation appearing in my scratch notes,
- ( 2 – x ) * R3 + x * r3 = 3.026
where “R” represents the yttrium radius and “r” the radius of barium. Solving for x yields the next equation in my scratch notes (written here on one line),
- x = ( 3.026 - 2 R3 ) / ( r3 - R3 )
Plugging in the ionic radii of yttrium and barium from Kittel yields the final equation,
- x(Y,Ba) = 1.4173 / 1.6560 = 0.856,
my initial estimate for the barium substitution, later truncated to 0.8 for the reasons described previously.
Resuming the reconstruction of the numbers in Meng’s notes, in retrospect (circa 1994), I discovered that I accidentally transposed the weights (the 0.1 and 0.9 figures above)[90] to yield the following equation,
- x RY3 + (1-x) RBa3 = 0.1 RLa3 + 0.9 [y RY3 + (1-y) RBa3].
Recreating this error is critical to reproducing the specific compositions.
At this point, I apparently had noticed a shortcut that would greatly simplify the final equation. As described in my dissertation, the origin of the (Y0.6Ba0.4)2CuO4 composition was linked to preserving the average ionic volume among the larger metal ions in (La0.9Sr0.1)2CuO4. Using this fact along with the nearly identical radii of La3+ and Sr2+ and the relatively small quantity of strontium,[91] I made the approximation,
- RLa3 ≈ 0.9 RLa3 + 0.1 RSr3 ≈ 0.6 RY3 + 0.4 RBa3.
Making the appropriate substitution in the above equation yields,
- x RY3 + (1-x) RBa3 = 0.1 [0.6 RY3 + 0.4 RBa3] + 0.9 [y RY3 + (1-y) RBa3].
Making the following substitutions,
- x = 0.6 + (x - 0.6)
- 1-x = 0.4 - (x - 0.6)
- y = 0.6 + (y - 0.6)
- 1-y = 0.4 - (y - 0.6)
allows one to cancel several terms on both sides, leaving
- (x - 0.6) (RY3 - RBa3) = 0.9 (y - 0.6) (RY3 - RBa3).
Canceling RY3 - RBa3 on both sides and solving for y yields the very simple equation
- y = [x - 0.6 (0.1)] / 0.9.
Note that the only non-unity numbers which appear in the final result are the 0.9 and 0.1 figures from the (La0.9Sr0.1)2CuO4 composition and the 0.6 figure from the (Y0.6Ba0.4)2CuO4 composition.
Inserting the values for x from samples YB-104, YB-105, and YB-103 (0.2, 0.3, and 0.4, respectively) yields,
- x=0.2 → y=0.15555..., 1-y=0.84444…
- x=0.3 → y=0.26666..., 1-y=0.73333…
- x=0.4 → y=0.37777..., 1-y=0.62222…
Note that the values correspond almost exactly to those of the LYB series samples listed above. The variation in round-off and truncation may have been due to using a borrowed non-RPN calculator (I did not take my HP-15C and am clumsy with the parentheses buttons) and recording rounded results of the intermediate calculations on paper between steps. Note also that when 0.6, one of the critical numbers in the (Y0.6Ba0.4)2CuO4 composition, is inserted, it reproduces itself, pointing to that composition as the focal point of the other compositions.
The shortcut mentioned above was only useful for the LYB series, but a shortcut for the LLB series can be derived from the LYB series results. Equating the Y-Ba and Lu-Ba average ionic volumes,
- y RY3 + (1-y) RBa3 = y’ RLu3 + (1-y’) RBa3.
Because the “R3” terms cannot be cancelled as before, the actual ionic radii must be utilized. Solving for y’ yields,
- y’ = [(RY3 - RBa3) / (RLu3 - RBa3)] y.
I did not take the textbook I had used in my solid state physics class (the original source utilized for ionic radii mentioned in my dissertation) to Houston, but this was irrelevant since it gave no standard radius for Lu (see image from Kittel above). Since I do not recall the precise source I would have used at this point, for purposes of the analysis, I will use the ionic radii from a widely available, familiar, and likely source that happens to include a radius for Lu3+, the CRC Handbook of Chemistry and Physics. Drawing on my vintage 60th edition (1979-1980)[92] yields the following where, for self-consistency, the CRC values for the yttrium and barium radii are also employed (repeating digits indicated by underline):
- y’ = [(0.8933 - 1.343) / (0.8583 - 1.343)] 0.155 = 0.148499,
a very near miss for rounding to 0.149.[93]
It should be noted that within the Meng notebook these samples are the very first after the initial discovery where raw material weights are formulated. Thus, they were presumably the first samples made after the initial YBCO discovery. In fact, by all indications they were made even before attempts in Houston to reproduce the YBCO materials made in Huntsville.
Summarizing the numbers in Meng’s notebooks alongside my reconstruction yields
- 0.155, 0.845 vs. 0.155, 0.844
- 0.26, 0.74 vs. 0.26, 0.73
- 0.378, 0.622 vs. 0.377, 0.622
- 0.149, 0.851 vs. 0.148499, 0.851501
With these figures as a reference, I would encourage the reader to compare the precision of my account with the remarkable imprecision of the other YBCO discovery stories examined in the pages ahead.
I do not recall the test results on these samples except that there was nothing of note. The point of these laborious derivations is simply that my story is firmly anchored to three significant digits multiple times over to compositions appearing in a Houston lab notebook. In addition, these compositions were clearly given very high priority in Houston in the frantic window between the 90 K discovery in Huntsville and the subsequent submission of the corresponding paper one week later. Any credible account of the discovery should reasonably be able to explain the existence and derivation of these compositions and should furthermore explain why the Houston team was, at least for a brief time, actually taking its direction, not from its soon-to-be “superstar-in-residence,”[94] but instead from a 22-year-old student from a small public university in north Alabama. Incidentally, at my request, Chu was questioned about the origin of these samples during the ongoing Hor-Chu case (see Appendix D). I have reason to believe his answer appears in his second interrogatory, but I am still (as of this writing) attempting to locate the document in the online archives. I can safely maintain that the answers will be found intriguing.
I will also mention here that very late in the writing of this manuscript, I came across a test among Meng’s notes that I had previously dismissed as being on a sample of yttrium-barium-copper-oxide. I have linked the test results here.[95] I had rejected as stray the leading mark in the sample identifier (reading it as YB-3) until I realized that there was no sample by that moniker. Thus, it seemed likely that the mark is indeed the letter “L” and the identifier is actually for LYB-3, one of the three-digit precise samples that I had formulated.[96] Note that the date of the test on LYB-3 is 30 January, the same as the test on the sample identified as “Wu’s #2” (the first test we ran on one of the samples from Huntsville – to be introduced later) and the page number (H810) of LYB-3 indicates that it was at least tested prior to “Wu’s #2” (H807), noting that the “H” numbers on the test results (assigned in the original patent interference case) are consistently in reverse chronological order.[97] My recollection is that several of my “three digit precise” compositions were actually tested before the first of the 90 K superconductors we had brought from Huntsville (consistent with the order in which they appear in Meng’s notes). At a later point in this narrative, I will provide information on how the reader might obtain copies of more of these test results (a process in which I am involved as of this writing).
If, on the other hand, one concludes that my tedious derivations and their otherwise coincidental relationship with the critical YBCO composition are simply a clever fabrication, then one must believe that within hours of the discovery, I concocted a plan whereby I would somehow coax the Houston team to make compositions other than that of the newly discovered “Holy Grail of Physics.”[98] Then, a year later, I convinced Robert Pool of Science Magazine of my role in the discovery, getting him to publish my scratch notes where I slyly added the cubed radii of yttrium and barium. At about the same time, I work additional scratch notes into the UAH Magazine. All of this, obviously, so that I could finally draw attention to them almost three decades later in this 200 page rant falsely claiming to have invented the first liquid nitrogen-cooled superconductor.[99]
Of course, Houston attorney Scott Chafin would be counted among those disputing my claim. In the ironically entitled article “Wu? Chu? Or Who?”[100] he was quoted as saying, “Dr. Chu suggested which materials and systems to research and test.” Given that everyone involved was making innumerable “suggestions,” this was, no doubt, literally true. The more pertinent question would ask whose suggestions were actually being “researched and tested” and, moreover, whose were successful. On the topic of irrelevant assertions, I would add Chafin’s continuation, “Wu might not have been involved in superconductors if he hadn’t been a student of Dr. Chu’s.” Obviously this, by extension, also applied myself, but since I really do not know where to start a discussion of how this supports Chu’s claim to YBCO, the analysis is left as an exercise for the reader.
Moving on with the story, the magnetic tests in Houston confirmed superconductivity at 90 K and a draft paper was assembled, largely by Chu.[101] In it, Chu cleverly transformed my attempt to preserve the Cu-O bond length into some kind of connection with his high pressure studies,[102] suggesting that the discovery was the result of an explicit decision to “investigate… multi-phase… compounds instead of the pure K2NiF4 phase.” In contrast, I have clearly demonstrated that the intent of (Y0.6Ba0.4)2CuO4 was to preserve the K2NiF4 structure, not replace it.[103] Of course, given the visible mix of green and black materials in the samples and “preliminary X-ray powder diffraction patterns” (second page of the original paper) already indicating the absence of any K2NiF4 structure, it was too easy for Chu to suggest that multiple phases and a potentially new crystal structure had been the intent.[104] For the reader unfamiliar with the concept of multi-phase materials, the footnote here[105] should be reviewed.
My timid protestations to Wu about the claimed pressure connection went unheeded as the joint publication listing the Huntsville group first was submitted, appearing in Physical Review Letters in early March.[106] The aforementioned “Yb typo” would be included (per the image of the galley proof above) but corrected before going to print.
February 1987
The narrative now transitions to points subsequent to the discovery, but before I get into some of the more substantial parts of the post-discovery period, this seems a convenient time for a side trip through one lesser facet of the story. It may be of interest to the reader as it is one prominent point where pure serendipity played a very significant role. Most subsequent accounts simply avoided this issue, perhaps because any emphasis on serendipity might have been counter to achieving other objectives. Nevertheless, it is a part of the chain of critical events and, in my opinion, is worthy of preservation.
As mentioned earlier, our work in the Huntsville lab had previously focused on metal alloys with low melting points. Thus, upon beginning our work with the copper oxide superconductors, we had relied on the previously-mentioned borrowed furnace, one whose analog controller simply displayed a maximum of three digits, that is, 999 °C. Thus, when the first YBCO sample went into the furnace, the temperature was actually about 100 to 150 °C below the widely preferred processing temperatures for the lanthanum-based materials that had been the focus of the superconductor community for weeks. We simply managed with the lower temperatures, although previously-mentioned fellow student Jason Kinzer would occasionally allow us the use of his hotter furnace. It was only later found that the pure-phase YBCO superconductor melts incongruently around 1025 °C. For the Y1.2Ba0.8CuO4 composition formulated and made first in Huntsville, incongruent melting would have been unfavorable.[107] Thus, the low furnace temperature (a limitation the Houston lab records suggest was not a constraint there) was quite simply a matter of blind luck.
A widely-held misconception by many of the groups working in the field was that the discovery was partly the result of a less favorable initial composition combined with overheating and incongruent melting, thus yielding samples with traces of the superconducting phase that were eventually isolated through a sequence of trial and error (consider Robert Hazen’s book to be covered in great detail in Part II). Interestingly, one such version of the story was related by James Gleick in an article from the summer of 1987:[108]
Dictated by Chu’s sense of atomic size; they mixed in the element yttrium for I.B.M.’s lanthanum. At first, the composition was all wrong. The furnace temperature had to be changed. But on January 29th…
To my knowledge, this is the only account that attempts to explain (erroneously) how the lower furnace temperature for YBCO came about. Incidentally, I do not know what a “sense of atomic size” is beyond the act of simply consulting a table of such values. As described previously with regard to Chu’s patent applications, he was not prone to use the more relevant numbers.
On the subject of the Houston patent applications, this is perhaps a suitable point in the narrative to cover the beginnings of the ensuing patent battle. Shortly after the discovery, both Huntsville and Houston independently filed patent applications. Some of what I report here regarding the applications is hearsay based upon knowledge conveyed to me later by UAH faculty and staff. However, in most cases, the information was corroborated by multiple individuals.
I was told that representatives of UAH, including one or more attorneys on retainer with the university,[109] caught up with Wu in Washington (convenient since that was where the law firm was located) sometime in mid-February. Apparently, Wu indicated that Chu had already filed an application on 12 January (mentioned previously) that covered the Y1.2Ba0.8CuO4 composition and that he (Wu) would somehow be made a part of the Houston claim (search for dollar signs later in this manuscript). Wu reluctantly yields to the authority of the university, a brief description of the invention is obtained, and a sketchy application is filed. However, it trails the 6 February Houston application by days, and, by virtue of the timing, UAH becomes the junior party in the patent interference to follow, putting the school at a decided disadvantage.
There is nothing of great significance in the UAH application. It attempts to patent a single combination of elements – yttrium, barium, copper, and oxygen, specifies a range of ratios, notes the now lower preferred processing temperature, and initially lists but a single inventor – M. K. Wu.
The Houston applications, beginning with 12 January,[110] take a somewhat different tack, listing the following (my emphases):
[L1-xMx]aAbOy
wherein L is an element selected from the group consisting of lanthanum, lutetium and yttrium, or a mixture of one or more of these elements;
wherein A is an element selected from the group consisting of copper, bismuth, titanium, tungsten, zirconium, tantalum, niobium, and vanadium or a mixture of one or more of these elements;
wherein M is an element selected from the group consisting of barium, strontium, calcium and magnesium or a mixture of one or more of these elements;
wherein x is a number in the range of about 0.075 to about 0.5,
a is a number in the range of 1 and 2,
b is 1, and
y is about 2 to about 4.
Given some knowledge of the history of oxide superconductors[111] combined with the fact that elements in a periodic table group tend to be chemically similar, it is not particularly difficult for one to discern most of the “rationale” behind the list of candidate elements.
The application goes on to describe that by replacing barium the “interatomic distances” are reduced (not preserved as had been my objective). The described processing actually includes quenching in an inert atmosphere, now known to be very unfavorable for the formation of almost all superconducting copper oxides.[112] The application then reiterates the importance of “reducing interatomic distances,” citing the previously-noted (and irrelevant in these ionic compounds) atomic radii of the various elements.
It is instructive here to consider how many combinations of elements are covered (a number that will increase substantially in subsequent applications). Excluding from each “mixture of one or more elements” the “null mixture” and considering that there are three L elements, eight A elements, and four M elements yields
- (23-1) × (28-1) × (24-1) = 7 × 255 × 15 = 26,775
different combination of elements, before considering the specific ratios or details of processing.[113] Chu will later mention in Robert Pool’s Science article from August of 1988[114] both his January 12th application along with reference to a “carefully planned substitution program.” I would presume that the patent application is at least somewhat reflective of that program.
For the sake of argument, however, assume that all single replacements (as opposed to “mixtures of one or more”) will be exclusively explored first in Houston, bringing the list down to 3 × 8 × 4 or a very manageable 56. In this way, one could conceivably arrive quickly at the composition responsible for the YBCO discovery (assuming the necessary ratios to somehow be known).
I must forewarn the reader here that the next several pages are an excellent example of how each aspect of this ensuing story leads one into yet another and another. I will eventually return to the main line of the narrative, but keep in mind that here in the chapter covering the first month after the discovery is the point in time that the situation begins to quickly degrade and a purely chronological analysis becomes all but impossible.
We now turn to a more complete set of pages from Ruling Meng’s notebook.[115] The page stamped H476 shows what appears to be a short-lived series of samples exploring some “new” elements and at least roughly, briefly, and very incompletely paralleling the “carefully planned substitution program” presumed to be outlined by the 12 January patent application, including:
- CL-1 and CL-2, (La0.9Ca0.1)2CuO4
- CeS-1, (Ce0.9Sr0.1)2CuO4
- CeS-2, (Ce0.85Sr0.15)2CuO4
- CeB-1, (Ce0.9Ba0.1)2CuO4
- CeB-2, (Ce0.85Ba0.15)2CuO4
- ML-1, (La0.9Mg0.1)2CuO4
- PL-1, (La0.9Pb0.1)2CuO4
- YS-1, (Y0.9Sr0.1)2CuO4
- YB-1, (Y0.9Ba0.1)2CuO4
- LuB-1, (Lu0.9Sr0.1)2CuO4
- LuS-1, (Lu0.9Ba0.1)2CuO4
The dates for these compositions can be narrowed to between the 7th of January (see the first column in the table on page H476) and the 18th of January (top of page H479). A second set of documentation from Meng[116] will show the raw material weights for some of these compositions being calculated on the 12th (starting on page H17) and 13th (starting on page H47) of January.
Of the eleven compositions listed above, only six actually follow the patent application formulas (cerium and lead are outside the scope), only the cerium samples appear to explore more than one set of ratios, and the fractional substitution of the second (divalent or potentially divalent) element for the first (trivalent or potentially trivalent) element never exceeds 15%. Furthermore, the preferred processing temperature for these samples is indicated as 1100 °C in air. Keep these conditions in mind as sample YB-1 is examined in more detail below.
The simple calcium substitution (CL-1 and CL-2) yields a superconducting transition between 30 and 16 K, below that of its barium and strontium analogs (note columns on the right of page stamped H476). A few more calcium samples will be tested later (see bottom of page stamped H479). Otherwise, after this short run in quite partial fulfillment of the “carefully planned substitution program,” work appears to refocus on the La-Sr-Cu-O and La-Ba-Cu-O materials (see the page stamped H477). The early deviation from the “plan” is hardly unexpected, as even the least competent of scientists is likely to refine his path with each new sample and test or else be faced with exploring thousands if not millions or billions of combinations at a pace of perhaps a half dozen per day.
Of course, of greatest interest here are the samples containing yttrium. The first, (Y0.9Sr0.1)2CuO4 (sample YS-1), is incidentally precisely the composition Wu will insist we try in Huntsville scarcely two weeks later.[117] Note that it is representative of the entire series where simple straight elemental substitutions (i.e., no or only minor adjustments to the ratios) are made for components in (La0.9Sr0.1)2CuO4 or (La0.9Ba0.1)2CuO4 (well-established as baseline compositions by then by producing consistently narrow transitions in the 30-40 K range) and using the same maximum processing temperature (1100 °C) preferred for those reference materials.
The second material, (Y0.9Ba0.1)2CuO4 (sample YB-1) hits the right combination of elements but is off-target on two counts. First, given what is, in hindsight, now known about the Y-Ba-Cu-O system, the barium substitution for yttrium (10%) is far too low to yield a material that would contain at equilibrium any portion of the 90 K superconducting phase. Second, again given what is now known, the processing temperature of 1100 °C is uncomfortably above the incongruent melting point of the 90 K superconducting phase (about 1025 °C) and the preferred processing temperature of no higher than about 950 °C. Thus, the elemental ratios combined with the high processing temperature would almost certainly have failed to produce a superconductor and would have been unlikely to yield even metallic behavior. In fact, this is precisely what is indicated in Meng’s notebook. Note the column on page H476 headed by the Greek letter “rho,” a symbol commonly used by physicists to indicate resistivity. Scanning down to the rows of the yttrium (and lutetium) samples one finds the resistivity of these samples denoted by the symbol “I,” meaning insulating, the worst possible result.[118] Scanning through the surrounding pages, one will note that such an unfavorable outcome was rarely explicitly noted. Sometimes metallic is indicated (“M”). Otherwise, the sample may be superconducting, in which case other details are indicated in the adjacent columns (transition start and end temperatures, general shape of the R vs. T curve, resistivity ratio, and magnetic susceptibility if measured). Thus, yttrium and the rare earths (at least lutetium) appeared to the Houston team to be likely dead ends and are abandoned until the “good news phone call” on January 29th.
Expounding upon what is now known about the Y-Ba-Cu-O system, sample YB-1 would have almost certainly consisted largely of what came to be known as “the green phase,” Y2BaCuO5.[119] As a general rule, materials that display “metallic colors” tend to be better conductors (and thus potential superconductors) while those that display “spectral colors” (“ROYGBIV”) tend to be insulators. Thus, sample YB-1’s color alone would have been very discouraging.
Chu’s recollection of the causes of the yttrium failures, at least as indicated in the August 1988 article in Science magazine, appears very different. He concedes trying and failing with yttrium in January (again, as part of the “carefully planned substitution program”). However, the writer of the article goes on to describe:
In one of those twists of fate that can make all the difference in history, Chu had used inexperienced undergraduate students to mix up the yttrium-containing compounds, and those batches showed no superconductivity… Chu had decided they were doing something wrong and had planned to assign a research associate to redo the substitutions. If the University of Alabama in Huntsville group had not found the yttrium material, Chu says, the Houston group probably would have soon.
Of course, based upon what was recorded (correctly, I presume) by the experienced Meng, it is clear that the actual cause of the failures was the selection of the wrong ratios and the wrong processing temperature. In short, Houston was changing but one variable at a time, an admirably systematic approach but, as has been demonstrated, an insufficient one. In fact, the resulting insulating samples can only be taken as an indication that whoever produced the samples did precisely as directed.
In any detailed description of a plate of spaghetti, it is always painfully difficult to decide which noodle to discuss next, and thus, for lack of a better place, I must insert this here. After the failures of all but the calcium samples, a few additional compositions are considered. Starting with the page stamped H18 in Meng’s notebook appear several pairs of compositions that bracket the compositions listed above (slightly higher and lower amounts of the M element substituting for the L element), including ones incorporating lead, calcium, lutetium, magnesium, and yttrium (pages stamped H18 through H21). Except for calcium, I can find no evidence that any of these additional samples were ever actually made or tested. However, I provide these patterns of bracketing compositions to explore another curious feature from Meng’s notes.
Consider again the (Y0.9Sr0.1)2CuO4 (YS-1) and (Y0.9Ba0.1)2CuO4 (YB-1) compositions from the list on the page stamped H476 (raw material weights appear on the page stamped H47). Meng’s 1990 declaration[120] includes an attached Exhibit A (page stamped H49) showing “bracketing compositions” for YS-1 and YB-1 similar to those for the other compositions listed above. The first yttrium-strontium combination is labeled YS-2. Its bracketing twin is labeled SY-3 (presumably the letters were accidentally transposed). The designator confusion seems to continue with the two compositions bracketing (Y0.9Ba0.1)2CuO4 as they are labeled SB-2 and SB-3 (presumably YB-2 and YB-3 were intended). The most curious part of this page, however, is the composition in the middle of these four, (Y0.6Ba0.4)2CuO4, the very composition responsible for the 29 January discovery.[121] A couple of items are worthy of note. First, the (Y0.6Ba0.4)2CuO4 composition appears as a very rare example of formulas without accompanying designators (recall that YB-1 is the insulating (Y0.9Ba0.1)2CuO4). Second, the date at the top of the page is redacted; this is actually acknowledged by Meng in paragraph four (page marked 2) of her declaration. She states that the date the composition was recorded was “prior to January 17, 1987.” By her declaration, (Y0.6Ba0.4)2CuO4 receives no more attention until the 29th of January (paragraph five).
Now fast-forward three years to Meng’s 1993 declaration.[122] Included with it is Exhibit F, a copy of the same page showing the strangely out-of-place (Y0.6Ba0.4)2CuO4 composition.

Exhibit F from Meng's 1993 declaration showing the suspicious (Y0.6Ba0.4)2CuO4 composition.
The first issue of note is that, now three years later, the redaction of the date has disappeared, now showing as 15 January 1987 (with enclosing box, presumably for emphasis).[123] Paragraph 17 of the much longer 1993 declaration (page marked 8) now notes the specific 15 January date. Again, the (Y0.6Ba0.4)2CuO4 composition receives no further attention until two weeks later (paragraph 18). I personally cannot even speculate why the date disappears and then reappears except to interpret this as indicative of the level of indecision in what story is to be presented to the patent court. As will be seen in Part II of this narrative, an even greater scale of waffling will appear in the stories presented beyond the patent court.
As I was writing this, it finally dawned on me why Ruling would identify the date as simply “prior to January 17th.” In my testimony in the patent interference, I stated that the critical YBCO formula came into being on the weekend of the 17-18 January, consistent with what I communicated to Robert Pool in his 1988 Science article (see page marked 656).[124] Thus, it suddenly made sense why Meng would conveniently confine the date of her YBCO formula to such an open-ended period. However, her redaction seems strangely unnecessary since what appears to be the original date[125] still preceded 17 January.
Resuming discussions of the Houston applications, several “continuations” to the 12 January patent will be filed by Chu, beginning with the 27th of January,[126] just one day before the Huntsville group will produce the critical YBCO material. The basic compositions and candidate elements remained unchanged. It is odd, given what Robert Hazen will later record in his book recounting Chu’s memories of the discovery, that nothing was refined in the January 27th application in response to the tantalizing hints of superconductivity supposedly seen in YBCO samples in Huntsville as the target composition was gradually refined (more on Hazen’s tale in Part II).
Among other reasons, my backtracking to the earlier patent applications is to lead up to the February 6th patent continuation,[127] filed within a week of the departure of Wu and myself for Huntsville. That application has some reasonably expected differences and at least one “unexpected” difference from its predecessors. To the list of “L elements” is added scandium (just above yttrium on the periodic table). After all, given what has been observed with yttrium, scandium might have seemed a reasonable addition.[128] The list of “A elements” is unchanged. However, it is the “M elements” that are most curious, having a single addition to the end of the list – mercury. Given over two decades of stories out of Houston, to my knowledge there has never been any mention of the mercury samples tested in Huntsville immediately prior to the YBCO discovery. What is further difficult to explain is that, while post-transition metal bismuth appears in the list of candidates to replace copper, its sister element mercury shows up instead on the list of “M elements” as a candidate to replace barium or strontium. The linked image highlights the degree to which mercury appears curiously misplaced among the various groups of elements.
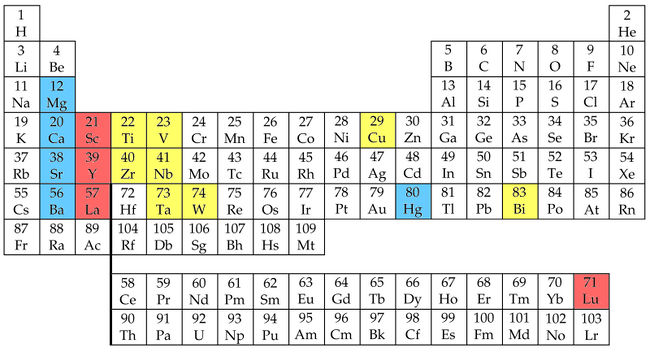
Periodic table highlighting the various groups of elements from the 6 February 1987 patent continuation. L elements in red. M elements in blue. A elements in yellow.
Of course, as I have previously documented, it was partial transitions detected near 40 K in samples containing mercury that were interpreted as confirmation of the ideas I was developing in Huntsville. The role of the mercury sample was communicated to Houston upon our arrival on the 30th of January. It was not until years later when I obtained copies that I discovered that mercury had found its way into the Houston patent applications.
Within weeks of the YBCO discovery, several groups including Houston will nearly simultaneously find that virtually all of the lanthanide elements are potential substitutes for yttrium. Thus, with the March 26th patent continuation,[129] Chu will, before scarcely putting a dent in the earlier “plans,” expand his “carefully planned substitution program” to cover 518,054,175 combinations of elements[130] (again, a tally not yet accounting for specification of ratios or processing), including the ubiquitous superconductor Sc-Y-La-Ce-Pr-Nd-Sm-Eu-Gd-Tb-Dy-Ho-Er-Tm-Yb-Lu-Cu-Bi-Ti-W-Zr-Ta-Nb-V-Ba-Sr-Ca-Mg-Hg-O and covering over one-third of the non-radioactive portion of the periodic table.[131] Incidentally, as of this writing, there are roughly 100 known “structurally or chemically distinct copper oxide superconductors” (to borrow Robert Cava’s terminology) after three decades of research worldwide. Given the great efforts required in finding and identifying so many of them, it is apparently fortunate for their discoverers that most were afield of the Houston patent application (by Hazen’s account, drafted in two days).
Resuming the story, most of the month of February was a blur. My recollection was that a press conference occurred some time in the middle of the month. I have an aging videotape but have not yet taken the time to digitize it. Not surprisingly, the local press descended on the UAH Science Building. Meanwhile, the national press converged on a parallel news conference in Houston. I vaguely recall some questions from the audience that created some slightly awkward moments between Wu and myself, and I more distinctly recall having the first realization that people might be genuinely interested in how the discovery really happened, unfortunately not a feel-good story about great leadership and a solid team effort.
March 1987
With the first week of March, the YBCO paper appeared in print. At that point, I assumed that proper credit for the discovery was secure, at least from external parties. At the same time, there was a growing body of evidence that I might face conflict from more nearby sources.
In mid-March, the American Physical Society hosted its spring meeting. The YBCO discovery prompted what came to be known as “The Woodstock of Physics.” Chu, and to a lesser degree Wu, are among the “featured speakers.” Ironically, one of the most widely published photos of one small segment of the crowd of over 2200 scientists will feature dead center the face of one very despondent graduate student, namely me.[132]
As the weeks passed, I became increasingly frustrated with the fact that Wu had felt it necessary to arbitrarily fuse my ideas with the pressure testing (see Media:140OptimalInterAtomicDistancesWu.pdf for some examples from that timeframe). Some time that spring (perhaps the end of March), Wu conducted a colloquium on the UAH campus (oddly in a liberal arts building, Morton Hall, as I recall; I still even remember roughly where I sat). In the course of his presentation relating the YBCO discovery, he stated that at some point leading up to the discovery, he directed me to make the Y1.2Ba0.8CuO4 sample but that I delayed a few days. When he followed this up with a chuckle, I was quite honestly on the verge of stepping down to the front of the lecture hall and physically assaulting him. I somehow maintained my cool but was so hurt and angry that I could literally not see clearly.
The next day, sensing that the YBCO discovery and all of its fallout were spiraling into a nightmare, I stood alone in the lab pondering the situation. There on the table just inside the lab door was Wu’s little-used lab notebook. On a hunch I began to thumb through it. With such sparse entries, it was not long before I discovered what I feared that I would (image below).
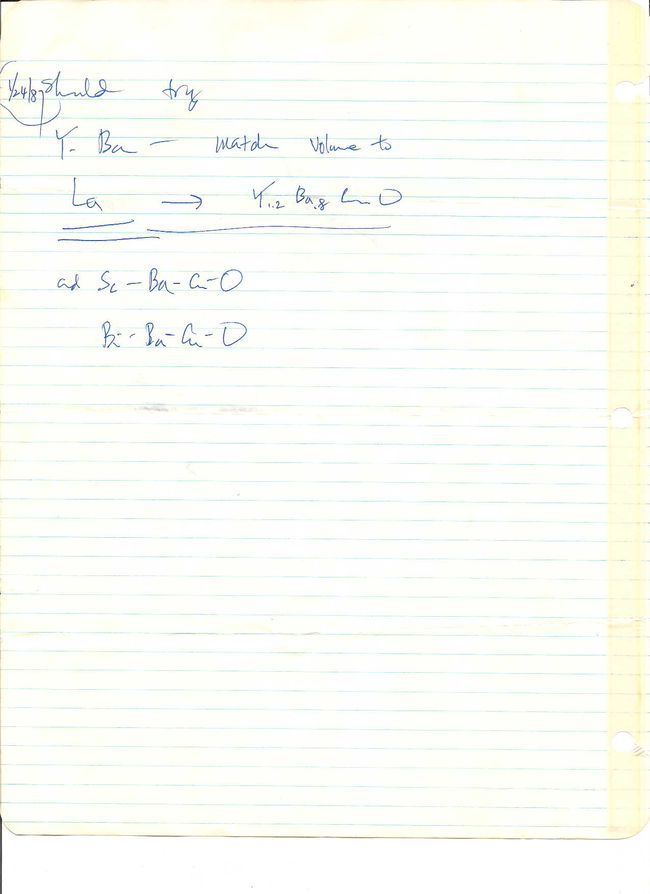
Photocopy of Wu’s backdated lab notebook page.
For what it was worth, I at least had something in Wu’s handwriting confirming how the discovery was achieved and debunking any "average ionic volume" coincidence theory (see Final Week of January 1987). However, in validating how, it was thoroughly muddying the question of by whom.
April-May 1987
On Monday morning, the 6th of April, I confronted Wu in the lab, and in a state of desperation, I took the liberty to covertly record our conversation. The entire track is linked here.[133] The first six minutes or so are particularly interesting and pertain directly to many of the facets of the discovery I have already covered. From the time I mention and then quote the lab notebook entry (at about the 2:15 mark), I prod Wu for over three minutes before he finally confesses (at about the 5:30 mark) to having added the entry after the discovery. Wu states in his defense something to the effect[134] that the UAH administration had instructed him to “get his books in order” in anticipation of the patent fight. Based upon my extensive work with the administration over the years to follow, I can proudly affirm that I was never asked to do anything deceptive, and I would never believe that doctoring lab notebooks was what they were suggesting.
The remainder of the track is certainly interesting on many counts.[135] One may note that Wu addresses several of the matters I have previously described. Most disappointing was the fact that Wu’s memory, scarcely weeks after the discovery, already seemed hopelessly confused. He had to be reminded that he had had so little faith in my ideas that he had insisted I make the failed Y1.8Sr0.2CuO4 sample, and he eventually seems content to conclude that I might have been the one who prompted all of the events that culminated in the discovery, but that he was somehow thinking all of the same things at about the same times. Incidentally, I had intentionally withheld from my subsequently published (1990) dissertation the details on why I averaged volume and not radius so as to allow some time for Wu to demonstrate that it was his thinking and not mine. Having afforded him an additional 25 years, this forum seemed the appropriate time to finally describe it. Another feature of note in the recording is that there is so little discussion of Chu or Houston. However, given that both Wu and I knew neither Chu nor his team had made any detectable contribution to the YBCO discovery, there simply seemed nothing along those lines to debate at the time.
Upon listening to the track, the reader will note repeated discussions of a particular paper. The paper to which Wu attributed great significance (and I do have the copy still somewhere within my files) included among its contents the Goldschmidt tolerance factor inequality that usually must be met to achieve a perovskite crystal structure. The formula did eventually find its way into my notes, and can be even be seen in the top margin of my first scratch notes page shown earlier. The number on the left side of the inequality was scribbled out because it is, at best, approximate, and I doubted the applicability of the commonly quoted values to the unique K2NiF4 “double perovskite” structure. I think that the obscured figure is 0.75. Using 0.72 Angstroms as the ionic radius of copper and 1.4 Angstroms for oxygen (per my chart) yields a potential range for the radius of the A ion (represented in my formula by R(La)) between 0.85 and 1.60 Angstroms. Thus, on my y-axis are two tick marks with arrows marking this span. On a later version of this chart these tick marks became lines across the chart, the lines to which Wu references. As the reader will note, this tolerance factor defines a range that accompanies at least of dozen elements (per my chart) and would do little to point the way to the critical Y1.2Ba0.8CuO4 formula. Like Chu, Wu seemed to be very prone to attributing excessive significance to events in which he had a hand, and, like Chu, the tolerance factor did no more to zero in on the critical formula than did Chu’s shotgun patent applications.
Beyond this, the reader may also note Wu’s repeated references to our “discussions.” In other words, he seems to concede that I was the prime mover but desperately clings to the idea that somehow, something he said to me somewhere along the way must have been essential to the discovery. Since “the paper” seemed to be, in his mind, his most significant input, then it is clear that his direct contributions to the YBCO discovery were, at best, negligible.
The reader might also note a third voice at some point in the audio recording. If I recall, I think one will hear me address the individual as “Daniel” before asking him to grant Wu and myself some privacy. In any case, that voice is of the Daniel Shultz I mentioned earlier. Wu hired him shortly after the discovery to help us with synthesis of the copper oxide materials. As a reminder, this is the high school friend who graduated from Georgia Tech with a degree in ceramic engineering and was the person who suggested I seek out a ceramist (Ed Ethridge, mentioned earlier, the only other ceramics specialist I knew) in my search for yttrium oxide. Of course, the reader is free to assume that the ceramics connections are all simply coincidences and that it was Meng who somehow knew that yttrium oxide would be the first reagent we would fail to find in the UAH chemistry stockrooms and would thus suggest we go straight to “NASA” (more on this in Part II).
In late April, the Materials Research Society hosted its semi-annual meeting in Anaheim. For the second time in a row, Wu attended without me. I will note here that the remainder of this paragraph is based upon hearsay communicated to me later by Rustum Roy. At the meeting, Roy[136] and colleague Amar Bhalla[137] of Penn State obtained consent from both Wu and Chu to be “interviewed” about the YBCO discovery. The interviews were designed to be interrogations, with the objective being the truth. As such, Wu and Chu were questioned in separate rooms. Caught off guard and unable to coordinate their stories, they were extremely evasive. However, there were apparently a few mentions of my role in the discovery (presumably by Wu). Roy, angered that he had failed to meet his objective, continued to follow the ever-evolving story in the media and was eventually prompted to write a series of letters to Science magazine addressing the aggressively propagated misinformation. Robert Pool,[138] a writer with the magazine, was motivated to investigate. After contacting Roy, he went to Penn State to view the videotapes, leading him to write the aforementioned August 1988 article. Science passed on publishing Roy’s letters, choosing instead to “break the story” on its own and make no mention of the tapes. In a later phone call (ca. 2000), Pool confirmed with me that he had seen the videotapes. The lone set eventually vanished, presumably lost during a relocation of the Materials Research Society offices in the mid-’90s.[139] Thus I was denied the opportunity to ever view them myself.
The spring of 1987 closed with some early preparations to attend a meeting in Berkeley dedicated to superconductivity.
An Intermission – Calendars and Such
Even as organizing this narrative to this point has been difficult, moving forward into and beyond the summer of 1987 is only more complex. The story transitions from one of discovery to more one of the many tellings and retellings of the discovery. To minimize the degree to which the story must often circle back and to simplify the process of following the evolution of various aspects of the story from beginning to end (the end being the time of this writing), I will instead move through the timeline more quickly, introduce a few of those retellings, especially as they are tied to timeline events, and then, in Part II, I will double back and explore the various accounts in more detail, less hindered by trying to maintain a chronological story.
Given my personal ground rules, I will open this section by violating them, but only because I will be dealing with more items that were supposedly penned before the discovery. Coming after the discussions of Wu’s modifications to his notebook, this point seems as good a place as any other. The earlier notebook entry had several features that I simply found, for lack of a better term, humorous, in a dark sort of way. The date on the page was circled (as if for emphasis), and the fact that the entry started with the words “Should try” seemed to me a bad joke. “Really?” I thought to myself. Surely I was not expected to believe the circle was original, and who would write “should try” in front of an idea for a new composition? To whose benefit would that serve? I wrote down dozens of such formulas over the course of that January, and it was never necessary to remind myself, “should try.” The fact that Y1.2Ba0.8CuO4 topped Wu’s very short list of “should try” candidates among the many other new compositions tested in the Huntsville lab (all apparently unworthy of an entry in his notebook) would seem just a bit too clairvoyant.
As a point of comparison, recall the copy of my pocket calendar linked earlier.[140] I have included in the scanned pages only those with entries; all others are blank. A perusal of its contents reveals the following entries:
- January 9th:
- Balance savings account
- Find checking acct. statement for December
- Travel estimates
- PH561 notes for Tues. & Thurs. (Jan. 6 & 8)
- Buy binder paper
- Check P. Chem. & Guy Smith’s labs
- Finish data
- January 10th:
- Oil change
- Thermos & Soldering Iron Tip (Weller TC202) & Strip-X
- January 12th:
- Check on account at Central [Bank]
- Make more samples
- January 14th:
- PH561 notes
- Check P. Chem.
- Pay for Tag
- Transfer money savings → checking
- HW [homework]
- January 16th:
- Order LHe
- Pay car tag
- Check on account at Central
- Oil change
- (Some entries in my auto mileage record)
There are no more entries after the 16th of January, and sadly, there is simply nothing about Y1.2Ba0.8CuO4 or even a passing reference to yttrium. With what would have seemed the most convenient and perhaps convincing spot in which to have such a reference, I am instead stuck mostly with entries on matters of personal finance and automobile maintenance. In fact, I can offer not a single dated document indicating the point in time where I formulated the critical Y1.2Ba0.8CuO4. I suppose that I am not particularly skilled at “getting my books in order.” However, I would like to think that reproduction of the previously discussed three-digits precise compositions from Meng’s notebooks, a most difficult place to which I might make after-the-fact edits, is somewhat more compelling.
On the subject of calendars, among the items that have come into my possession in recent years is a copy of Paul Chu’s desk calendar, one that his wife supposedly rediscovered years later.[141] The pages for February and March (points after the discovery) look much like any from my own calendar above – “to do” items, reminders, abbreviated notations, short notes, and messy or scratched out entries. December and January, on the other hand, are very different. There are at least twenty-five past tense entries, one can only presume to have been entered after the events noted (the reader will be left to speculate as to precisely how much later). Examples include (italics for emphasis and items in square brackets are mine):
- Asked Wu to join project
- They gave Kitazawa opportunity to present his results
- Called CY [Huang] MK Wu for Michel-Raveau paper
- Told GTH [Ted Geballe?] results not encouraging
- Talked to David Voss very encouraging
- Decided to go to PRL
- Called [Myron] Strongin (positive but f[illegible])
- Called [Neil] Ashcroft [Brian] Maple } APS Meeting
- Muller called & thanked
- Banquet speaker – announced > 50 K
- Decided UH [press?] release on 1/30/86
- Agreed to send F[illegible] a sample
- Worked on UH [press] release
- Failed to contact Zhao
- Told THG [Ted Geballe] all details of sample preparation and results
- Talked to Zhao… should retract, 70 K record is ours (UH)
- Chaudhari – called [illegible]
- Late PM TGH [Ted Geballe] – measured our sample [illegible]
- Horgen Sci American called
- Gubser called – MRS Conf.
- RW called
- C. T. Wang[?] – MCC called
- NY Times called – record is ours!
- Observed Tc>77 K
- Wu arrived at 10 am
On the February and March pages, in comparison, I personally could not locate a single past tense verb; however, the reader is certainly encouraged to try his own hand and to perhaps estimate the causes of this curious shift in conventions.
Chu’s calendar pages devote a great deal of attention to press coverage,[142] even over the course of the two months prior to the discovery:
- UH Press release on Jan 5/87
- NSF [National Science Foundation press] release
- Decided UH [press] release on 1/30/86
- Rec UH [press] release
- Worked on UH [press] release
- Send [sent?] Release to RW .F. Exp. New Hampshire! O. U.
- Press Conf 2 pm Hou[ston] Post, [Houston] Chronical [sic.], NY Times
- Business Wk, Radio[?] sts[?] & More
- NY Times about Chinese result[s?]
- Discovery article[?]
- NY Times called – record is ours!
- Weinstein press rel, patent
However, the most interesting aspect of the pages is that they have been referenced by Chu as proof of his “conception” of the YBCO superconductor (see the discussion of the “Bannerot letter” later in this narrative). Prior to the 29th of January (the date of the YBCO discovery in Huntsville and subsequent “good news” phone call), yttrium has twice as many appearances in Chu’s calendar (four) as there are yttrium samples shown as tested in the Houston lab notes (the two mid-January failures covered previously):
- December 18th:
- [illegible] complete replacement of La by smaller Y, Yb, Lu,…
- December 19th:
- no Y…
- December 26th:
- Y, Lu have to work, [illegible]
- January 2nd (a very faint entry, for whatever reason, and difficult to read)
- Think about patent
- Cu – Nb, Zr[?], V[?], Ta[?], W, [illegible]
- La – Sc, Y, Yb, Lu
- Ba – Sr, Ca
The January 2nd entry certainly seems credible given the contents of the 12 January patent application (the “shotgun approach” covering transition metals from known superconducting oxides for copper; “nonmagnetic” rare earths for lanthanum; and non-radioactive alkali earths for barium). With all due respect, “Y, Lu have to work,” which incidentally appears after one of the many past tense entries, seems rather odd given how quickly samples with these elements were produced, tested, found to fail, and abandoned in mid-January. Furthermore, one can only wonder how confident was Chu that the other 26,775 combinations from the January 12th patent application “had to work” (or the half billion from the March application).[143] Concerning the December 18th entry, it is extraordinary, if not unbelievable, to think that Chu could have anticipated that only complete replacement of lanthanum with yttrium was best for achieving 90 K superconductivity, especially given that his patent applications specifically covered mixtures at every point in the formulas.[144] In any case, these being the only entries of “should-try” elements and the fact that yttrium leads off three of the four of them (all of the darker entries) would, like Wu’s single entry, seem incredibly prophetic, especially when combined with “have to work” and “complete replacement.” Incidentally, Chu’s December and January calendars read much like an outline for a couple of his papers from the mid-’90s recounting the YBCO discovery, right down to his note to send a postcard on the 14th with a reminder to express his excitement about the prospects for superconductivity at higher temperatures. Amazingly, it was this very postcard that was returned to him later (presumably only after the YBCO discovery) to be included in those later papers.
I would recommend here a mental comparison of my lengthy volume-matching derivation (with corroborating formulas from Meng’s notebooks and my scratch notes) to Wu’s doctored lab notebook entry and Chu’s supposedly recently-discovered desk calendar with the “Y, Lu have to work” entry. For reasons the reader is left to discern, I go to astronomical lengths to validate what is arguably only circumstantial evidence in support of my story of the conception of the Y1.2Ba0.8CuO4 formula while both Wu and Chu seem to have relatively unambiguous and dated evidence in support of their claims. Of course, if I am truly pushing a fraudulent claim, would it not have been much easier for me to simply add an entry to my pocket calendar?
At this stage, I will move more quickly through the timeline for the next 28 years (up to 2015), after which Part II will explore various topics in even greater detail.
June-December 1987
(Author’s Note: Please pardon the change of tense. For reasons I cannot quantify, I had to surrender after repeated failed attempts to convert the remainder of Part I to present tense.)
From the 22nd to the 26th of June 1987, I attend a meeting at the Marina Marriot in Berkeley, California. After Monday’s opening and welcome, Chu follows Karl Müller (codiscoverer of the first copper oxide high-temperature superconductor) with a 9:45 a.m. presentation entitled “The Discovery and Physics of Superconductivity above 90 K.” The corresponding paper in the Novel Superconductivity proceedings becomes the first of many confusing and ever-evolving accounts of the discovery. It also marks the beginning of what I have christened “the legend of sample J-31.” J-31 may very well be the most amazing aspect of the discovery story retellings – much more on this subject in Part II.
In July of 1987, Chu is widely reported in the national press[145] [146] [147] [148] as discovering superconductivity around 240 K in another oxide of yttrium, barium, and copper. According to the articles, Chu reports “unstable… earmarks of superconductivity at about the temperature of dry ice,” leaving him “optimistic, however, that the results would eventually be reproduced.” The test data of interest appears in a paper innocuously entitled “Superconductivity above 90 K.”[149] The chart (Figure 2 on the page marked 4682) appears to show at least three additional transitions at temperatures above 90 K – one about 175 K, another around 220 K, and another curious drop around 240 K. Accompanying the chart, Chu writes (my emphases):
Finally, it should be pointed out that resistive indications of superconductivity at 120, 150, 180, and 240 K have been detected many times by us as shown in Fig. 2 as early as February 7, 1987. Unfortunately, they are not stable enough to survive the thermal cyclings for us to carry out further diagnostic checks… As mentioned earlier, it took about 2 months (November 25, 1986,[150] to January 29, 1987) to stabilize the 90 K superconducting phase. It is not unforeseeable to take another few months to stabilize the superconducting phase with a Tc at 240 K.
While Chu’s report was good for considerable levels of press coverage, for whatever reason, not even one of these three higher temperature superconducting Y-Ba-Cu-O phases has, to my knowledge, made a reappearance since the summer of 1987, and this despite the fact that Y-Ba-Cu-O is almost certainly now among the more studied systems in history.[151] In my opinion, Chu should perhaps consider more closely monitoring the out-of-phase signal during AC resistivity measurements; it is a reliable indicator of bad electrical connections where a single loosening current lead can easily make for multiple phantom superconducting transitions.
Concerning other superconducting phases that actually have been identified in the Y-Ba-Cu-O system, here are a few, none of which superconducts above 100 K:
- YBa2Cu4O8 (80-84 K)[152] [153]
- Y2Ba4Cu7O14 (40-65 K)[154] [155]
- Y3Ba5Cu8O18 (93+ K)[156]
- Y7Ba11Cu18Oy (94 K)[157]
In September of 1987, I am added as a coinventor on the UAH patent application. I know neither how I was omitted in the first place nor specifically what prompted the correction.
By the fall of 1987, I am seriously questioning my career path. It had always seemed that the thrill of discovery was one of the driving forces for pursuing a science profession. Given that assumption, it was unclear what was I to do given that the thrill had been replaced with terror. Most of the next two years are spent in a haze. I slog through my classes, still managing to make straight A’s, but tend to avoid the lab (and especially Wu) as much as possible.
1988-1989
Late in the evening of March 29th, 1988, I am flipping through the channels on the television when I catch a lead-in for a NOVA program on superconductivity. I pop a cassette in the VCR and call high school friend John Brown – “John, I’ve got to get out.” We happen to end up at the remake of D. O. A. with Dennis Quaid and Meg Ryan. The reader is encouraged to survey on the web some of the more complete synopses of this movie to understand how that day was perhaps the lowest point of my life. More on the “dramatic NOVA reenactment” in Part II.
In July of that year, Graeme Duthie, the UAH Physics Department chairman, pens a letter to The Scientist.[158] In it, he writes (my emphases):
The actual discovery of the first material to become superconducting in liquid nitrogen was wrongly attributed to Professor Paul Chu of the University of Houston. The discovery was made on January 29, 1987, at the University of Alabama in Huntsville. Dr. M.K. Wu and his students came to the decision to try the Y-Ba-Cu-O material based on some ideas on the ionic radius of the components. They prepared the material and made the initial discovery of a transition to zero resistance at liquid nitrogen temperatures. After seeing their results, the Huntsville team knew they needed to observe a transition in the magnetic susceptibility of the material to confirm that they were, indeed, seeing superconductivity. Lacking the necessary equipment, they called Dr. Chu and made arrangements to use the University of Houston’s facilities. Dr. Wu flew to Houston that night and confirmation of the magnetic susceptibility transition was made the next day in Houston.
Dr. Chu is a senior scientist and, in fact, was Dr. Wu’s PhD. dissertation adviser. Partly because of his seniority and the relative size of the University of Houston to the University of Alabama in Huntsville, the press has given Dr. Chu credit for the discovery. The fact of the matter is, however, that the thoughts leading to the first liquid nitrogen superconductivity and the actual first observation of the effect were made in Huntsville, not Houston.
Note that Duthie gets it right – ionic radius, not atomic radius.
Over the course of the year, Chu,[159] and to a lesser degree Wu, wins several national and international awards for perceived contributions to the YBCO discovery.
In August, Robert Pool writes the aforementioned Science magazine article[160] outlining some of the controversy growing between UAH and Houston. Incidentally, had I not happened to be the one to answer the phone when Pool called the lab seeking Wu, then the story would likely have been a dead end. Realizing at the time that Wu might be bolder given that he had just informed me of his recently-accepted tenured position at Columbia, I coach Pool on the best questions to ask.
By the spring of 1989, upon returning from the Spring MRS Meeting in San Diego and supported by my new advisor Elmer Anderson (who routinely challenged me to some very therapeutic racquetball sessions[161]), I begin to climb out of my abyss. To survive mentally, I take up running (up to five miles a day), dropping 27 pounds by year’s end.
Since 1989
In the fall of 1990, I make a hard push to complete my dissertation, defending it in November and walking in December, facing no pushback from my committee, the department, or the School of Science when my acknowledgments recognize Wu for simply “introducing the author to the field of superconductivity.” There were similarly no inquiries when in Chapter II (“Discovery of Superconductivity at 93 K in Y-Ba-Cu-O”) I wrote (my emphasis):
This chapter will address the question of the motivation which led to the discontinuous leap in compositions from the 40 K superconductor (La0.9Sr0.1)2CuO4-y to the 90 K superconductor (Y0.6Ba0.4)2CuO4-y. The ideas discussed were conceived and developed by the author during the two weeks prior to 17 January as a diversion to research on (La,Sr)2CuO4-y.
Having already taken a job that previous summer far from superconductivity, pure research, and academia, I continue to be stalked by the fallout from the discovery. Over the next few years, the patent interference between UAH and Houston accelerates. Despite all my attempts to push it from my mind, the legal proceedings will provide frequent unpleasant reminders.
By the mid-1990s, the patent interference is at full speed. I spend too many horrific hours dissecting the Houston documentation. Far and away the bulk of the testimony (declarations, depositions, lab notebooks, test results) is given by Ruling Meng. The Houston account will simply erase any and all contributions by the UAH team to the critical path, defer the “discovery” to 1 February (when the first Houston-made samples are tested, see paragraph 21 of the Meng declaration to be linked later), and conveniently omit from the evidence the test results on Huntsville samples done in Houston on the 30th and 31st of January. I will not obtain copies of those latter results until 2011. One example is included here.
Resistivity vs. Temperature of sample marked “Wu’s #2,” one of the UAH samples tested in Houston during the period 30-31 January. All results on samples marked as originating in Huntsville were withheld during the UAH-Houston patent interference.
Meng’s documents from the original case not linked elsewhere in this manuscript are linked here.[162] Among my correspondence with the UAH patent attorney is a reconstruction of the likely actual sequence of events in Houston as well as exhaustive analysis of the inconsistencies in Meng’s testimony.[163] My analysis will be somewhat superseded when, in the Hor-Chu Case (see Appendix D),[164] Meng will deliver to the Federal District Court in Texas a March 2006 affidavit confessing to perjury in the earlier YBCO patent interference. This document will be covered in more detail later.
In 1996 and 1997, Chu publishes a series of similar papers giving some of his most detailed accounts of the discovery. Oddly, despite coming almost ten years after the event, these accounts will easily be among the most accurate coming out of Houston. Almost ten years after the Hazen book, Chu will finally move the discovery back to Huntsville and repin it to the 29th of January. Note that these papers were not authored until after the patent discovery period closed. In any case, I will not discover them until the summer of 2000, only months after the patent court’s final decision. The “residual errors” in these accounts will be covered in more detail in Part II.
On the tenth anniversary of the YBCO discovery, with the UAH faculty and staff now almost universally aware of the role of one 22-year-old graduate student in the discovery, the university erects a marker on the wall outside the former superconductivity lab:[165]
AT THIS SITE a major advance in superconductivity was achieved on January 29, 1987 by Dr. Jim Ashburn who, as a UAH graduate student working with Prof. Maw-Kuen Wu of the UAH Physics Department, discovered that a material composed of Yttrium-Barium-Copper-Oxygen (YBCO) would superconduct at 93 K, more than doubling the previous record and surpassing the technologically-significant 77 K temperature of liquid nitrogen.
The final hearing for the patent case is conducted during the summer of 1998, by my recollection. According to the patent attorney at the time, Steve Kelber, the hearing went very well,[166] and he was cautiously optimistic of a victory. The final decision uncharacteristically takes almost six months. According to Kelber, one of the three judges had quit and another retired in the period before contributing to the final decision. No transcript is taken during the final hearing (an option at that time and chosen to manage costs). Thus, the two replacement judges are left with only the written record.
In early 1999, I receive a phone call from one of the staff attorneys from UAH informing me that the patent court had decided in the favor of the senior party Houston based upon the conclusion that the oxygen content of our samples fell outside of the scope of the application. My immediate response was, “Are you sure you have the right patent?” New to the case, the replacement judges ignored all of the mountains of documents and based their final decision on a completely uncontested issue, failing to understand that the oxygen ratio was essentially an unknown quantity that was only estimated and was fixed by the processing conditions. Examples in the Huntsville notes where the oxygen ratio was simply omitted as shorthand (and one ill-placed typo in my declaration) prompted the decision that we had made, quite impossibly in air or oxygen, Y1.2Ba0.8CuO1. The third judge consents but writes separately, inviting a request for reconsideration (essentially a “mini-appeal”).
Within a year, the court awards Bell Labs a patent on the pure-phase YBCO superconductor (YBa2Cu3O7), the “black specks” in our mostly green mixed-phase samples. About six different organizations had isolated the superconducting phase within a matter of days of each other. Four of them, including Houston, had become entangled in the subsequent patent fight. Despite the “coincidental” timing of the four groups’ “discoveries,” the patent court will still conclude that the identification of YBa2Cu3O7 was an “invention” worthy of a separate patent. The pure-phase patent effectively trumps the earlier patent on the mixed-phase material, thus rendering it effectively worthless.[167]
Just days before the deadline for filing the request for consideration, I receive a call from Kelber’s boss, Chico Gholz. Gholz informs me that, as he was working over the weekend, Kelber came into his office on a Sunday morning and announced that he had quit, effective immediately (Kelber recently informed me via email that his “departure from Oblon was announced more than a month in advance” and that he “left with five other attorneys from Oblon to go to Long, Aldridge, and Norman”). Gholz stated that he had been sifting through Kelber’s papers trying to figure out what most needed attention. The finishing touches on the request for reconsideration are hastily applied and the document is filed. It is subsequently denied, throwing any subsequent actions to the Federal District Courts. In the intervening period, I discover the mid-’90s papers by Chu. Now armed with the new accounts by Chu that completely discredit the story previously offered by Houston to the patent court, UAH files an appeal, partly on principal and for my benefit given the fact that the patent now appears to be largely worthless. Houston files a counterclaim, accusing me of all manner of unethical actions and maintaining that I was, from the legal perspective, strictly a “pair of hands.” UAH, having already sunk about $500K into the fight, agrees to a settlement. In the course of the negotiations, UAH staff attorney Mike Spearing informs me that Houston would be willing to pay for certain concessions that in my understanding would hinder my ability to challenge or assist others to challenge the inventorship. I tell him no. He responds with something to the effect of “You don’t want to know how much first?” I respond by giving him specific instructions to pass along to the Houston attorneys concerning the best location for their money.[168] Spearing suggests that I am an idiot[169] and the phone call ends. Apparently my priorities are atypical, and I can only presume that the message was translated before delivery.
Meng’s “perjury affidavit” appears in 2006 (more on that in Part II). In 2007 and 2008, Chu makes a couple of presentations recounting the YBCO discovery and continuing, two decades in, to perpetuate the story of sample J-31.
In November of 2011 and after exchanging a couple of emails in search of a digital copy of her program, I inform Linda Garmon (director of the NOVA episode on the discovery) that her reenactment of the YBCO discovery was entertaining but otherwise pure fantasy. She never replies to my last message.
In January of 2012, the Alabama Historical Commission erects a marker outside of Wilson Hall (former home to the UAH School of Science) acknowledging a “graduate student” for the YBCO discovery. The commission does not customarily include the names of living persons on their markers. I am perfectly content to keep my name off for as long as possible.
In the months leading up to May 2012, I exchange several emails with Francis Galasso and his colleagues Steven Suib and Treese Hugener-Campbell concerning the role of Galasso’s aforementioned book in the discovery. Campbell informs me that Galasso was much encouraged by my “news” in the wake of his wife’s passing in August of the previous year.
In August of 2013, I send a hand-written letter to Chu in an attempt at some kind of reconciliation. Now over a quarter of a century into this nightmare, I have found that I tend to make copies of everything:

Letter from Jim Ashburn to Paul Chu, August 16, 2013.
After about eight total phones calls (from December 2013 to about September of 2014) and several emails, I have yet to speak with him. I never held out much hope of a nonconfrontational end to this saga, but without a chance to speak one-on-one, the chances would seem to be precisely zero.
Part II, The Evolution of the YBCO Story
Before an even more in-depth examination of the YBCO stories, it is helpful to try to enumerate the factors that had to converge for the discovery to take place. I will simply adopt as a matter of expedience the conventions employed in Chu’s 12 January 1987 patent application. Recall:
[L1-xMx]aAbOy
…wherein x is a number in the range of about 0.075 to about 0.5,
a is a number in the range of 1 and 2,
b is 1, and
y is about 2 to about 4.
Thus, the variables of interest are:
L, an element, usually a rare earth and most commonly lanthanum
M, an element, usually an alkali earth metal but occasional other metals
“x,” a number that describes the degree to which M replaces L
“a,” a number that, since “b” is one, one can regard as the ratio of the total amount of L and M to A (presumed to be copper)
“y,” a number, the oxygen ratio which is a function of the processing, both the temperature[170] and atmosphere
Therefore, to explain how Y1.2Ba0.8CuO4, or equivalently (Y0.6Ba0.4)2CuO4, came into existence, one must explain why:
- L was chosen to be yttrium
- M was chosen to be barium
- x was chosen to be 0.4
- a was chosen to be 2
- the maximum processing temperature was in the range of 900 and 1000 °C
- the final heating was in an oxygen-rich atmosphere of air or pure oxygen
I have offered previously in this narrative my explanation for how these conditions converged (and established its credibility to a substantial degree of numerical precision). It is now instructive to examine alternative accounts, taking each of the above conditions one or two at a time.
Element L
Concerning the element represented by the variable “L,” almost all of the samples preceding the YBCO discovery, both in Huntsville and Houston, were based upon lanthanum (thus the particular letter chosen by Chu to mark its place). In discussing patent applications, notebook entries, and calendars earlier, I have covered to some degree the topics of who, when, and how regarding the selection of yttrium. To those, I will add the following.
From D. R. Clarke’s “The Development of High-Tc Ceramic Superconductors: An Introduction”[171] (emphases are mine),
In retrospect it appears that a number of the groups were making a straightforward substitution of Y for La under the assumption that the compound Y2CuO4 analogous to La2CuO4 exists.
Just 30 pages later in the same collection, Roth et al. write (again, emphases are mine),[172]
Despite the protestation of solid state chemists that Y+3 and Ba+2 could not substitute randomly for each other, many physicists attempted to form K2NiF4 type phases of the sort “Y2-xBaxCuO4.”
For a reference to a specific group, consider this from A. Khurana,[173]
News about superconductivity at temperatures up to 100 K in an oxide of yttrium, barium and copper also appeared in the People’s Daily (China, 25 February) and, John Rowell of Bell Communications Research told us, prompted researchers at Bellcore to measure the resistivity of an oxide of yttrium that their magnetic measurements had earlier suggested was very likely not superconducting. “A set of five samples containing different ratios of yttrium and barium were prepared on 3 January, but x-ray measurements showed these to have multiple phases.”
To Roth, I would reply that my specific intent was, in fact, to eliminate random ordering of the first two elements, which I did achieve (see my dissertation copy for more on this subject). A second example appears in the 1988 NOVA documentary “Race of the Superconductor” (to be covered later in this narrative) where the University of Tokyo reported retesting in response to the Huntsville/Houston announcement their earlier Y-Ba-Cu-O samples and detecting magnetically trace amounts of the superconducting phase. Hazen in The Breakthrough describes the frustration of the Tokyo group as expressed at the March 1987 American Physical Society meeting (page 237):
The Tokyo spokesman told of a month of frustration synthesizing ‘many green stuffs,’ because the furnace temperature was slightly too high.[174]
In any case, given the above quotes, it should be no difficult leap of the imagination to conclude the following:
First, yttrium, as a candidate, was universally obvious to those in the field (although rejected after consideration by some). Thus, any claims on the “idea of using yttrium” are meaningless, if not comical.
Second, because it was so obvious, it is no stretch to speculate that most, if not all, of the members of both Huntsville and Houston teams did, at some point prior to the discovery, in some sense, independently “think” of the “idea.” Thus, it comes as little surprise that so many (including, at a minimum, Chu, Wu, Hor, myself, and perhaps others[175]) have asserted at some point that they were (or may have been) the stimulus for the yttrium efforts and subsequent discovery (see Appendix D for more on this topic).
Third, despite the fact that yttrium was obvious, Chu continued to find it necessary to repeatedly emphasize the role of his pressure measurements in pointing the way to smaller atoms. In his June 1987 Novel Superconductivity paper (mentioned a couple of times previously and finally linked here[176]), he offers his first written account, albeit brief, of his perception of the chain of events by which the YBCO discovery supposedly came about:
From our high pressure results, we found that smaller atoms tend to form a high temperature superconducting phase more easily. La (in Ba-rich LaBCO) is the largest among all rare-earth elements. It was, therefore, very natural for us at Houston and Alabama to try to synthesize mixed phase A-Ba-Cu-O compounds with A=Y, Yb and Lu which all occupy the same column in the periodic table and are not magnetic.
Oddly, after stressing the role of pressure in directing the search to smaller ions, he inserts the oddly worded line, “La (in Ba-rich LaBCO) is the largest among all rare-earth elements.” First, given that, in Chu’s own words, there was no direction to go but smaller, the role of the pressure measurements is clearly overstated. Second, concerning “in Ba-rich LaBCO,” the La3+ ion is the largest rare earth ion[177] independent of the compound in which it finds itself. However, the parenthetical does seem to serve the purpose of implying a means by which several pieces of the YBCO puzzle might have simultaneously converged.
Finally, from the above failures by Bellcore and Tokyo, not to mention the mid-January failures by Houston, it is clear that simply trying yttrium was hardly the distinctive condition that brought about the YBCO discovery.
Element M
I will move now to the second component “M,” usually an alkali earth metal (barium, strontium, and to a lesser degree calcium). The Huntsville team, prior to the YBCO discovery, tested very few samples containing barium, with attention almost exclusively devoted to strontium. In my above account of the events leading up to the discovery, I described in great detail why, upon replacing lanthanum with yttrium, I simultaneously reverted from strontium to barium. In contrast, Meng’s notebooks suggest that the Houston team, again prior to the YBCO discovery, gave roughly equal emphasis to barium and strontium, with earlier attention to barium and a slight trend towards strontium moving into January. Chu’s Novel Superconductivity paper gives this explanation in the text immediately prior to the above passage:
One of these samples was a Ba-rich LaBCO coated with a dull-pink insulating layer. Without perturbing the sample, we decided to test its magnetic properties. On January 12, 1987, we observed a large diamagnetic shift in this sample, starting at ~100 K and reaching ~4 K a value of ~40% of that for a bulk superconductor, as shown in Fig. 2 [Ba-La-Cu-O J-31]. Unfortunately, the diamagnetic signal disappeared the next day. These observations strongly convinced us that superconductivity above 77 K must exist and the only question left was how to stabilize the high Tc phase.
Figure 2 will be examined in greater detail shortly as I examine in greater detail the credibility (or incredibility) of J-31, the most prominent and pervasive piece of legendry from Chu’s YBCO discovery stories.
Numbers x and a
I will next cover the elemental ratios “x” and “a” together, again turning to Chu’s Novel Superconductivity paper as the starting point. It has been seen twice in the above excerpts the emphasis on “Ba-rich.” Unfortunately, Chu never (here or in any subsequent account I have ever been able to find) sees fit to mention the specific ratios in sample J-31 (again, more on this later). This would initially seem odd given that, upon examination of Meng’s notebooks (page H473), one finds that the formula for J-31 is (Ba0.4La0.6)5Cu5O5 (last subscript is in error; should be 15 less some small delta), which can be reordered and renormalized to (La0.6Ba0.4)CuO3, meaning that “x” (the portion of yttrium substituted with barium) is a perfect match to that of the composition yielding the YBCO discovery. Initially, one can only wonder why Chu would repeatedly omit the detailed formula, initially, that is, until it is noticed that the “a” value in this composition is one, whereas “a” was 2 in the Huntsville YBCO formula. In fact, “a” was 2 in about 78% of the Houston tests prior to the discovery and over 98% of the Huntsville tests prior to the discovery. However, in both labs, a combination of a high value for “x” (>0.2) and an “a” value of “2” appears to have never occurred in either lab prior to the YBCO discovery. I have included here a couple of graphics demonstrating how the YBCO discovery composition was very much an outlier. Whereas I have offered my detailed explanation above, Chu, on the other hand, seems to rely more heavily on implications and strategic omissions.
Diagram depicting samples made and tested in Huntsville through 28 January 1987.
Diagram depicting samples indicated as made (and some tested) in Houston through 28 January 1987.
For reference, the x-axis corresponds to the value for “a” and the y-axis corresponds to the value for “x.”[178] I have included on the chart for those familiar with more conventional phase diagrams, some of the now-known tie lines in the Y-Ba-Cu-O phase diagram mapped from the ternary diagram into these rectangular coordinates. The thinner contour lines represent increasing amounts of the subsequently-identified pure-phase 90 K superconductor YBa2Cu3O7. Note that the phase diagram details are only meaningful when the combination of elements is specifically Y, Ba, Cu, and O. The overlay of the mostly lanthanum (and often strontium) based compositions is simply to illustrate where those compositions might have fallen given the correct combination of the first two elements (yttrium and barium). Note from the contours that tie lines emanating from YBa2Cu3O7 represent “ridges” whereby a composition might yield relatively higher proportions of the 90 K superconducting phase without necessarily having a composite composition visually close to YBa2Cu3O7.
With that, I will concede here the fact it was mostly serendipity (see the latter part of chapter 2 of my dissertation for an analysis of this) that my Y1.2Ba0.8CuO4 formula fell so close to the top of the “ridge” between YBa2Cu3O7 and the so-called “green phase” Y2BaCuO5 (represented by the point at [3, 33.3%]), thus yielding a sample that was roughly one-fourth by volume the YBCO superconductor and three-fourths the relatively benign insulator Y2BaCuO5.[179]
Temperature
Next, concerning the processing temperature, I discussed that similarly serendipitous aspect of the story earlier (see section entitled “February 1987”). Note from Meng’s notebooks that maximum processing temperatures in Houston typically ranged from 900 °C to (not uncommonly) 1100 °C (or even higher).
Atmosphere
Finally, concerning the processing atmosphere, the Huntsville lab consistently used air or flowing oxygen. This condition reliably produced the best results in the lanthanum-based materials. Within the Huntsville lab, a reducing atmosphere was never considered. An oxygen-rich atmosphere[180] is now universally known to be most conducive to formation of the pure-phase 90 K superconductor. Returning again to Chu’s Novel Superconductivity paper, the previous excerpt made reference to “these” samples. From the lines immediately prior, one finds (emphases are mine),
Many mixed phase samples were subsequently made under different conditions and tested for superconductivity. Some of these were purposely synthesized in an oxygen-deficient environment in the hope that a wide range of phases could be formed in one sample. One of these [J-31]…
With the curious exception of the processing atmosphere, Chu seems to be trying to pull together in sample J-31 (albeit not without a struggle) most of the factors that might culminate in the discovery. However, J-31 is worthy of much closer scrutiny. For that reason and even after so much discussion, I will assign J-31 its own dedicated chapter to follow shortly.
Favorable Conditions
I will close here with a table summarizing some of the compositions of note fabricated in Houston and Huntsville. With the obvious exception of the Y1.2Ba0.8CuO4 sample that was the basis for the 90 K discovery, all of the other samples miss the favorable conditions by at least two factors:
| L=Y | M=Ba | x=0.4 | a=2 | 900-1000 °C | air or O2 | |
| Houston Y1.8Ba0.2CuO4 (YB-1) | ||||||
| Houston Y1.8Sr0.2CuO4 (YS-1) | ||||||
| Houston (La0.6Ba0.4)5Cu5Oy (J-31) | ||||||
| Huntsville Y1.8Sr0.2CuO4 | ||||||
| Huntsville Y1.2Ba0.8CuO4 |
Table showing the degree to which various samples of interest fulfilled the conditions favorable for detecting 90 K superconductivity in YBCO. “Yes” indicates that the condition is fulfilled. “No” indicates the condition is not met.
Recall that Chu’s “carefully planned substitution program” (to whatever degree it was indicated by his 12 January patent application) included 26,775 combinations of elements, even before one includes the final four columns of this table. Needless to say, the systematic approach was unlikely to get the Houston lab to 90 K superconductivity very quickly if ever, suggesting that a shortcut was necessary. I have described the shortcut as I remember it (recall three digits precise compositions from Meng’s notebooks as my proof). Now I will explore Chu’s supposed shortcut to 90 K superconductivity.
Sample J-31
I personally “discovered” for the first time sample J-31 in Chu’s Novel Superconductivity paper.[181] Given that there was no one physically closer to the sequence that climaxed in the YBCO discovery, it is strangely ironic that I should only learn about J-31’s supposedly pivotal role over four months after the event.
In examining J-31, I will begin by tracing its many appearances over the years in Chu’s YBCO discovery accounts (at least those I have found), starting with what I believe to be the original from the page marked 583 in Novel Superconductivity. For convenience, I have included an image of it here:
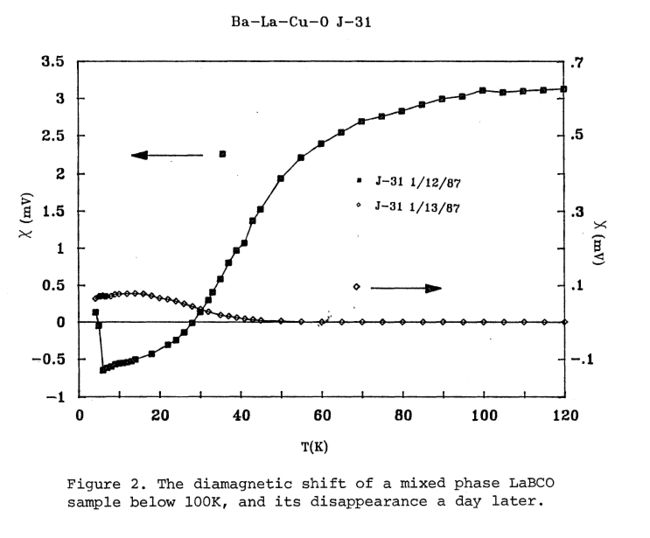
Chu’s first published graph of the magnetic susceptibility test attributed to Houston sample J-31, as appearing in Novel Superconductivity, June 1987.
The first curve (12 January) that starts high at high temperatures and ends low at the lower temperatures[182] is described by Chu as evidence for diamagnetism (a property of superconductivity) in sample J-31 starting around 100 K where the curve appears to begin to fall. The second curve, according to Chu, indicates that the superconductivity had mysteriously vanished the next day (13 January).
Fast-forward to 1996 and a paper by Chu entitled “Superconductivity above 90 K and Beyond.”[183] Figure 4 on the page marked 21 shows the supposed diamagnetic signal from J-31 now shifted down to start along the x-axis. The figure has been reproduced here:
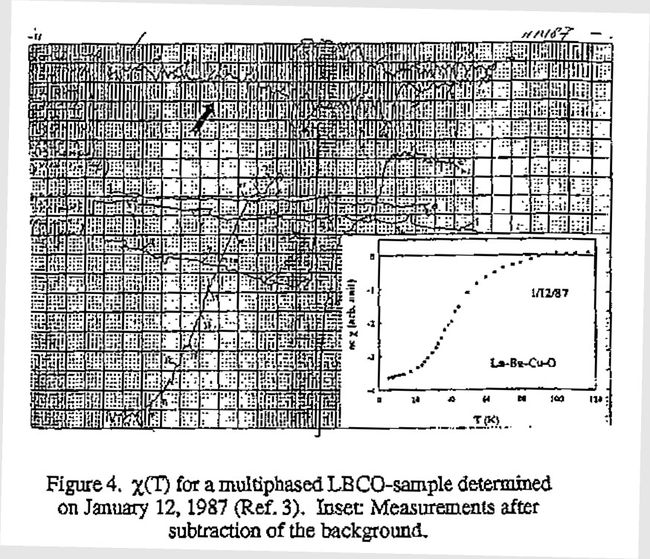
Data attributed by Chu to sample J-31, as appearing in “Superconductivity above 90 K and Beyond.”
In the background is a fuzzy image of what Chu suggests is the original 12 January data with an arrow pointing at what is presumed to be the start of the transition around 100 K. For the inset, the caption notes “Measurements after subtraction of the background.”
That same year, the chart appears again on page 809 of a paper by Chu from History of Original Ideas and Basic Discoveries in Particle Physics.[184] An image of the graph is included here:
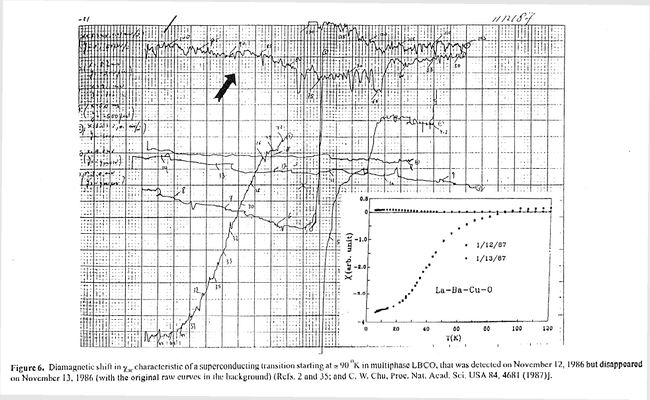
Data attributed by Chu to sample J-31, as appearing in “High Temperature Superconductivity.”
The image shows a much larger and clearer version of its predecessor. I will note here that the dates in the caption mistakenly read “November 1986” instead of “January 1987.” The dates on the foreground chart are correct. Again, a large arrow appears to draw attention to some salient feature in the original data.
Following J-31 into the twenty-first century, it reappears in 2007 in a presentation by Chu in Denver.[185] Page 13 shows the J-31 chart (background again supposedly subtracted). Ironically, the aforementioned photo of me from the Woodstock of Physics appears on page 2 and again on page 25, sandwiching charts devoted to press clippings (page 9), patent applications (page 12), and even the previously-mentioned 14 December 1986 postcard (page 10).
A variant of the Denver presentation is shown in Urbana later that same year with J-31 on page 12.[186] Incidentally, the first page of the body of the presentation quotes from Mark Twain:
What is it that confers the noblest delight? What is that swells a man’s breast with pride above that which any other experience can bring to him? Discovery!
My personal experience with discovery is that it tends to confer many emotions, none of them pleasant, however.
Again J-31 appears in 2008 in Saint Louis (see page 17).[187] This is perhaps a good point to take this higher resolution image and overlay it with the first version from the Novel Superconductivity paper:
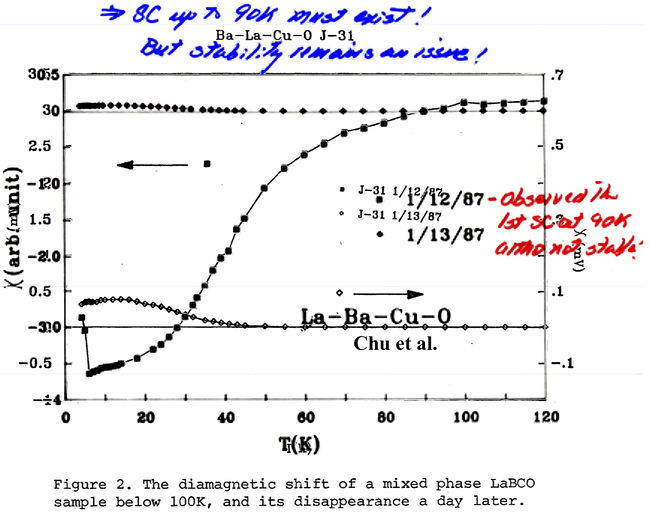
Overlay of J-31 magnetic susceptibility charts from Chu’s Novel Superconductivity paper (1987) and APS Saint Louis presentation (2008).
Yes, the two January 12th curves are on the chart. It would seem that, curiously, the “background” was determined to be precisely 3.0 mV and perfectly temperature-independent. Obviously, the curve was simply vertically offset by a convenient round number so that the curve would start near zero at high temperatures. But what if there actually was a background measurement? I will return to that subject shortly. My main point so far (before digging ever deeper) is that J-31, along with the drama of its supposed instability, is the most prominent part of Chu’s YBCO stories – from June 1987, where it is presented as the key and final stepping stone, to as recently as 2015.[188]
As a point of reference for the examination of J-31, consider a good magnetic susceptibility test. The example I will use is taken from Figure 2 on the page marked 909 in the original YBCO paper.[189] An image of the chart appears here:
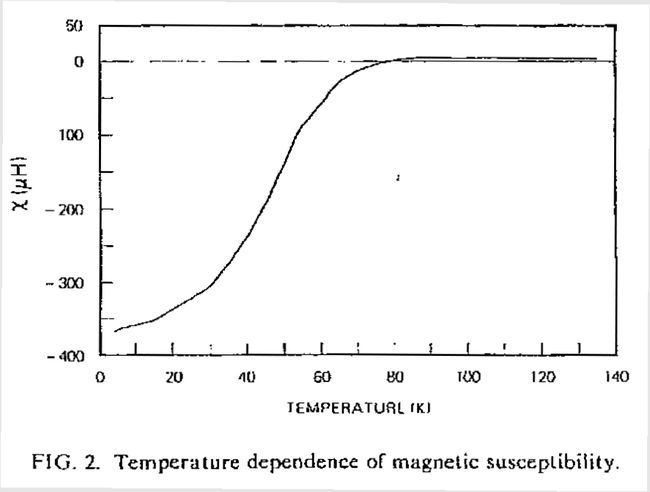
Magnetic susceptibility test results on one of the earliest YBCO samples. As appearing in Physical Review Letters.
This test, to my recollection, was conducted on 30 January 1987. Furthermore, my recollection is that the results as shown in the original paper are consistent with the raw data,[190] including the extremely flat and near-zero data above 90 K, indicating that any background signals have been properly compensated. Overall, the YBCO and J-31 charts (at least the “background subtracted” version for the latter) certainly look very similar.
In the weeks following the discovery, it was quickly discovered by Houston (and others) that yttrium in the 90 K YBa2Cu3O7 superconductor could be successfully replaced by most of the lanthanide elements to produce an analogous structure and yield superconductivity near 90 K (thus, Chu’s 26 March 1987 patent application includes the lanthanides). The J-31 data, if found to be credible, would certainly suggest the possibility that Houston had actually made a sample containing some of the lanthanum-based cousin of YBa2Cu3O7. This would be somewhat historically significant as it would move the initial date of the discovery of superconductivity above the boiling point of liquid nitrogen (albeit, by Chu’s assessment, “unstable” and clearly not in a sample containing yttrium) from 29 January 1987, presumably in Huntsville, to 12 January 1987, now in Houston. As will be demonstrated, however, it appears that J-31 is less a product of science and more one of… imagineering.
Fortunately for the science historian, the raw data relevant to reconstruction of the 12 January 1987 J-31 results is available. So, I will now walk through that process. Using the figure assigned to J-31 in the History of Original Ideas and Basic Discoveries in Particle Physics paper, one can consult Meng’s lab notes to find an even better image of the raw data.[191] Turn to the page marked RLM0584 and take a moment to note the matching date, shapes of the curves, and sample number (partially cut off in the top left of both images). A reproduction of the much clearer raw data is included here:
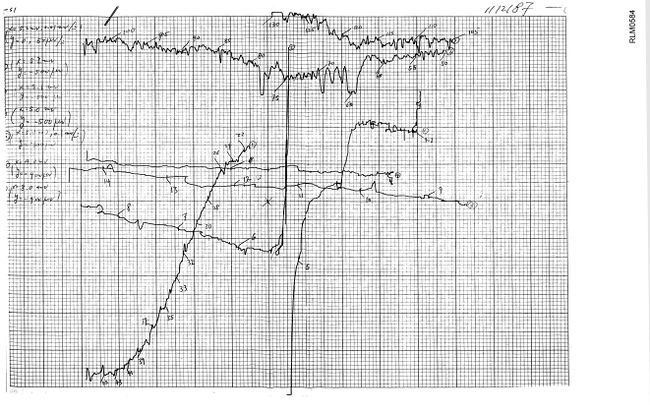
Magnetic susceptibility results on sample J-31 results as taken from Meng’s copies.
I described early in this narrative the process by which Wu and I collected measurements prior to my automation of our testing. The Houston lab at the time still collected data with a plotter[192] in the manner Wu brought from Houston to Huntsville. Each line on the chart is numbered, and along the side is shown the offsets to and scales for the x and y coordinates that must be applied to each segment to build the composite curve (e.g., the foreground charts from Chu’s images).[193] Fortunately, in many of the Houston charts, someone has already read the data and converted the x-coordinate from thermocouple voltages to the corresponding temperatures, as shown by the short tick marks connecting numbers (Kelvin temperatures) to the segments. Thus, to build the final result, one only needs to note the temperature for each point, read the y-coordinate, and apply the necessary scale and offset. The scale for each axis is normally indicated beside the first recorded offset and then updated only when it is changed. Going through the process (the reader is highly encouraged to check my work) produces the following chart, complete with black arrow pointing to the presumed salient feature:
Reconstruction from raw data of January 12th, 1987 sample J-31 magnetic susceptibility results.
I would urge the reader to be patient here. While the reconstruction looks nothing like the 12 January results that first appeared in print in Novel Superconductivity and the arrow appears to point to quite simply nothing of significance, I must in all fairness emphasize that the reconstruction process is not yet complete. For their magnetic susceptibility tests, it was the convention in Houston to perform a background measurement immediately before or after that of the sample under examination. The tests were typically labeled “Background” or equivalently “Empty Probe.”
Roll back from Meng’s 12 January J-31 chart by a single page to the sheet stamped RLM0583. The date is partially cut off but is the same day.[194] An image of the chart is included here:
Magnetic susceptibility results labeled “Background” as taken from Meng’s copies.
Applying the reconstruction process mentioned above and subtracting the resulting background[195] data from the 12 January J-31 data yields a curve that I have conveniently overlaid on the original Novel Superconductivity chart:
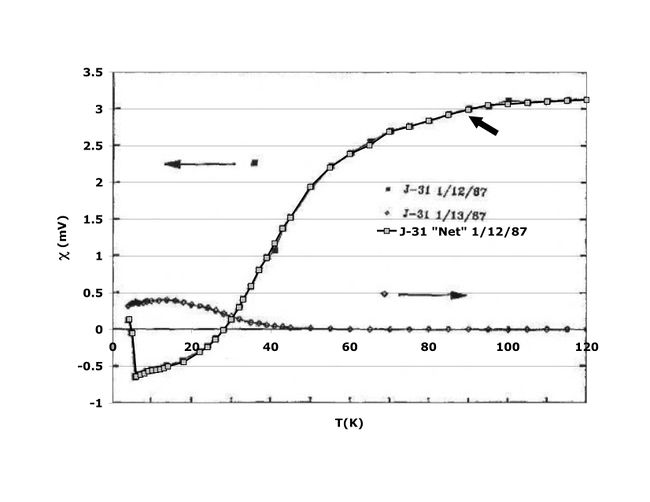
January 12, 1987 University of Houston magnetic susceptibility measurement of J-31 with background subtracted.[196]
First, I would suggest that the quality of the match indicates that I have faithfully reproduced the process by which Chu’s 12 January J-31 graphic was generated; most of the small differences would be attributable to how different individuals might read numbers from the moderately noisy plotter data. Second, I must point out that the reconstructed chart already has the background subtracted. Thus, Chu’s later versions of the J-31 results with the precise 3 mV vertical offset appear to be arbitrary adjustments that serve only to bring the graph in line with what is to be expected from a good test (refer to the results shown from the early YBCO test above[197]), among those properties a negligible net signal at temperatures above the critical temperature of any supposed superconductor. Clearly the lingering bias at higher temperatures in J-31, both in the original Novel Superconductivity chart and in the current reconstruction of that chart, is present in the original data and is worthy of scrutiny.
I will interject here that the 3 mV adjustment to the J-31 results is not the only such case I have examined where Houston data was arbitrarily adjusted by a convenient round number. Sample #1b, another ubiquitous but less historically significant part of many of the discovery stories, is analyzed in Appendix A of this narrative.
At this stage, an astute reader’s curiosity might be aroused concerning the details of my final step in the reconstruction process. To that end, I provide here a single chart showing the 12 January J-31 test (sample plus background), the 12 January Background test, and the calculated difference representing the net signal from J-31 with the background subtracted:
January 12, 1987 University of Houston magnetic susceptibility results including “J-31” (sample plus background), “Background,” and “J-31 Net” (sample signal with background subtracted).
The underlying results, if I can use an idiom, do not paint a pretty picture. Relative to the J-31 sample plus background signal (which is relatively small), the background signal is quite large, so large, in fact, that the features of the supposed net signal are completely dominated by it. As a point of reference, if one were to chart the net signal as a function of the background signal, an R2 (coefficient of determination) of 0.993 is found. In comparison, a similar chart of the net signal as a function of the J-31 “sample plus background” yields an R2 of only 0.413. The bottom line is that the shape of the curve attributed to J-31 is determined almost entirely by the background measurement, a background with a large signal that appears to be absent when the test was conducted with sample J-31 actually in the chamber.
The next day (13 January), J-31 is tested again. Chu’s Novel Superconductivity rendition shows the results as unremarkable (except for the supposed disappearance of superconductivity). The background in the 13 January test is presumably already removed, netting the expected near zero signal at higher (>45 K) temperatures and an overall small signal when one notes the much tighter secondary y-scale. Later versions of the chart (shown above) put both curves on the same scale, further emphasizing the supposed disappearance of superconductivity.
In 2012, I emailed Hor, one of the original members of the Houston team, and he confirmed this observation.[198] Just as he describes dismissing the results on J-31, Meng’s notebooks suggest that she arrived at the same conclusion (at least at the time), that J-31 was unexceptional. On the page stamped H473 in her notebook,[199] the line recording J-31 lists nothing indicative of superconductivity at all (transition start and end noted as 0 K, resistivity indicated as insulating, and no entry in the magnetic susceptibility column).
Assuming the validity of my J-31 analysis is conceded, I suspect that it will be advanced that Hor failed to properly inform Chu of the problems with the testing. However, the complete testing on J-31 actually spanned at least nine days, including several background/empty probe tests. Anyone who failed to arrive at the conclusion that the results were, at a minimum, suspect was clearly either out of touch with the activities of the Houston lab or simply selectively interpreting the test results. Furthermore, the fact that J-31’s net signal failed to level off very near zero at higher temperatures was an immediate red flag, and an arbitrary 3 mV adjustment to “fix” J-31’s problems is unconscionable.
So, was there was any evidence for a 100 K transition in the first place, even if only in the background test? The answer is no. Note on my reconstruction overlaid on the Novel Superconductivity chart the minor discrepancy at 100 K. Having carefully read and reread the original data (both sample and background), my curve, which shows a smooth trend from 120 K all the way through and beyond 100 K, is correct. The Novel Superconductivity version, however, has a curious blip at the 100 K point. The single blip lends to the illusion of an actual transition at 100 K that is all but perfected when offset in later renditions to trace the x-axis at higher temperatures.
Incidentally, outside of the University of Houston, there has never to my knowledge been a report of superconductivity at 100 K in La-Ba-Cu-O. In 1988, Wada et al.[200] observed a transition to zero resistance in LaBa2Cu3O7-y starting at 93 K and ending at 92 K. Their very narrow transition suggested near optimal processing conditions.
So if there is no actual transition at 100 K, what is causing the background? One should note here that the bane of these kinds of measurements is ferromagnetic contamination. Ferromagnetism (what the layman associates with the word “magnetism”) is a potentially much stronger effect (as much as six orders of magnitude) than the diamagnetism associated with superconductivity; thus, even the tiniest of particles can present serious problems. Ferromagnetic contaminants combine proportionately large signals (both intrinsic and induced) with hysteretic effects to yield all manner of unexpected and unpredictable behavior in a magnetic susceptibility test.[201] In the lab, nonmagnetic tweezers and other such tools are used to minimize this contamination, and while the risk is ever-present, a little attention to the background measurements is a reliable countermeasure. I would also note here that ferromagnetic materials typically yield broad smoothly-varying increases in magnetization below the so-called Curie temperature, curves often described as scaling as 1/T until saturation at some sufficiently low temperature[202] and a trend consistent with what was observed in the 12 January J-31 background measurement.
So, beyond some potentially unidentifiable source of ferromagnetic contamination, is there possibly a more ready source? Wada et al.[203] (mentioned above) emphasized the requirement for a low-temperature anneal in oxygen as the final step towards superconductivity in LaBa2Cu3O7 above 90 K. However, J-31 was processed in a “reducing atmosphere” (specifically 32 hours in vacuum as indicated in Meng’s notes, page stamped H473). Recall also J-31’s “dull-pink insulating layer” (from Novel Superconductivity), “red” according to Meng’s notes (page H473 again) and the labels atop the 15 January tests. The evidence would seem to point to the presence of non-stoichiometric cuprous oxide (Cu2O), specifically cation-deficient, meaning stoichiometrically between Cu2O and CuO. One might review the work of Chen et al.[204] and consider the following.
Processing. The starting material that supplies the copper to J-31 is CuO. It is heated to temperatures in the range of 1000 °C in a vacuum (well above the temperatures required to reduce the oxygen and effectively move the composition closer to Cu2O). Incidentally, lanthanum is routinely numbered among the most difficult rare earth elements from which to form the ~90 K superconducting “123” structure,[205] and that even with the knowledge of the necessity of low-temperature annealing in oxygen.
Appearance. Cuprous oxide is sometimes used as a pigment in antifouling paints, specifically red paints. It is typically a dull-red color.
Electronic and magnetic properties. Depending upon the stoichiometry, the resistivity of cuprous oxide can cover a wide range. At a minimum, however, it would be described as semiconducting, consistent with Chu’s description of J-31’s insulating surface layer. Finally, cation-deficient cuprous oxide has been reported as weakly ferromagnetic below 455 K.[206] Thus, that the suspected 100 K transition never actually flattens out but continues trending above and beyond 100 K with smoothly decreasing slope is consistent with the presence of Cu2-xO contamination. Finally, the strong net signal at higher temperatures (prior to the second background removal) is decidedly not superconductivity and is most reasonably assumed, at a minimum, to be spontaneous ferromagnetism.[207]
So, does what is known now about these materials support Chu’s theory of unstable superconductivity? While references in the literature to instability in copper oxide superconductors are plentiful, these must be understood in their context. In many cases, a theorized crystal structure is noted as unstable as an indication that it will not form in the first place without, for example, introducing some partial substitution of one element for another. Other references cite instabilities associated with reactions with the environment, most commonly water in the form of humidity. There is also a sense in which all of the copper oxide superconductors are inherently unstable; however, as the referenced article clarifies:[208]
While this does not mean that these ceramic compounds are useless or will break down at room temperature, it explains why they are so hard to make.
In other words, other crystal structures, not necessarily superconducting, are often otherwise preferred by the same combination of atoms. Thus, as materials are cooled, they tend to eventually find the most stable arrangement. Hence, all less stable arrangements are, in a sense, unstable. Outside of publications originating in Houston, I am at a loss to locate information (reliable or otherwise) concerning irreversible low-temperature crystal structure changes in the copper oxide superconductors.[209] We at the Huntsville lab only witnessed “unstable” results when electrical connections were “unstable,”[210] thermocouple reference junctions were adrift, or the like. In contrast, the accounts out of Houston are rife with the theatre of unstable materials, all of which appear to have a lifetime just long enough for one or two tests. It is unclear why such issues seem to plague certain labs much more than others.[211]
From page 14 of Chu’s Denver presentation in reference to J-31, one finds (my emphasis) “Although not yet pure and thus unstable.”[212] Oddly, Chu suggests some kind of fundamental relationship between impurity (i.e., the presence of multiple phases) and instability. Given that granite is a familiar multi-phase material that is not customarily associated with instability, Chu may need to provide more details on his insights into this particular topic.[213]
Based upon an early presentation by Chu in March of 1987 (see Appendix B), I strongly suspect that the 12 January sample and background tests were cross-labeled. Since Chu references the follow-on test where the superconductivity disappeared, I imagine that a closer examination of the associated background test, upon its availability, would be instructive.
From the body of the Houston notes, I suggest that J-31 may have also been first tested before 12 January, perhaps as early as 7 January. As of this writing, I have not yet obtained copies of the full set of test results, but I have found that they may be on file at the Federal District Courthouse in Houston, Texas as part of the ongoing Hor-Chu case (4:08-cv-03584, see Appendix D). The energetic reader should look for Exhibit 419 (H707-1133, Lab Notebook Entries), a trial exhibit advanced by Chu and admitted on 13 January 2014 (Transcript #184 makes reference to Exhibit #419) that should cover tests on the copper oxides in Houston from late November 1986 into early February 1987.[214] Once in hand, the reader should look for pages ranging from about 7 January to about 15 January, noting tests marked with “J-31” and roughly concurrent tests marked “χ - Background,” “Background,” or “Empty Probe.” One should also be aware that sometimes the polarity in a test may be reversed but that this can often be discerned by noting the polarity of the transition on the lead reference around 7 K. Temperatures can be determined for otherwise “unread” charts by using other “read” tests to determine thermocouple voltages at various temperatures. The reader should specifically look for recurrence (or likely absence) of the unusually large background signal associated with the 12 January test. I anticipate that the reader will also find that efforts with J-31 (including attempts to reproduce it) will end with mid-January, as the Houston team (or Hor, at least) confirmed that the 12 January test was suspect and the samples deemed unremarkable and unworthy of further examination.
The takeaway from this analysis of J-31 is that there is little to no evidence for any real 100 K transition, that it is uncertain whether or not what has been interpreted as a 100 K transition is even diamagnetism (i.e., evidence for superconductivity), that it is uncertain that the supposed 100 K transition can be attributed to an actual sample (instead of a measurement artifact), and that ultimately the evidence itself (with its interpretation) seems to be far more unstable than any superconductivity that might be present.
In closing this section, I have included one last undated rendition of the J-31 data that is an attachment to Meng’s 2006 “perjury affidavit:”
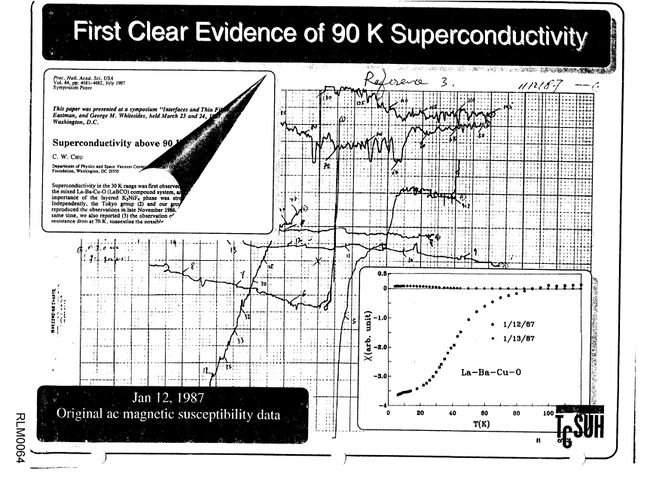
Chart depicting J-31 raw data (background) and non-matching processed data (foreground) declaring the results as “First Clear Evidence of 90 K Superconductivity.”
I would concede that the story of J-31 is indeed “clear evidence” for something. Unfortunately, that something is not superconductivity at or near 90 K. At best, it is evidence for a vivid imagination at room temperature. In reality, the “First Clear Evidence of 90 K Superconductivity” came on 29 January 1987 in Huntsville, Alabama, which brings us to some less technical issues, issues such as “when” and “where” and the like.
Who, What, When, Where? The Houston Chronicle
Given that I have rather hammered the questions of how and why the critical YBCO composition was first made, I will turn now to some more basic (and hopefully, for the reader’s relief, less technical) questions. A pattern of taking great liberties with the fundamental elements of the story (who, what, when, where) appears to have been established early and emerged in print a mere two-and-a-half weeks after the 29 January event.
On February 16th, 1987, an article appeared in the Houston Chronicle entitled “Discovery May Earn Billions, Nobel for UH,”[215] setting the stage for decades of “interpretive history.” In defense of science staff writer Carlos Byars, his principal source, Houston’s Dean of Natural Science and Mathematics, Roy Weinstein, could only best be described as overly zealous.
One particular stretch near the middle of the article is unprecedented in its embellishments. The sentences will be examined in sequence beginning with the following:
Weinstein says the competition involves three major issues: The discovery at an International Business Machines Corp. lab in Zurich, Switzerland, of the combination of chemicals used to make the [La-Ba-Cu-O] superconductor; the actual production of a superconductor from that material, which was first achieved at the University of Houston…
While Weinstein felt that Houston was the first to actually produce a superconductor from the La-Ba-Cu-O system, the Nobel committee that met in 1987 concluded that Georg Bednorz and Alex Müller’s paper “Possible High Tc Superconductivity in the Ba-La-Cu-O System” was sufficient for the Physics Prize. The above list continues:
…and most important, Chu’s discovery of the role of pressure in superconductivity, now called the “Chu effect.”
To my knowledge, Roy Weinstein is the only person to ever credit Chu with discovering the effect of pressure on superconductors or to refer to the phenomenon as the “Chu effect.” Despite his efforts, the moniker never stuck. Quoting Weinstein, the article continues:
“IBM-Zurich found some promising material. They didn’t know how to make it a superconductor or how to vary it to get a superconductor, but they did get everybody started in the right direction.”
Dr. Weinstein may be the only person who knows how the material showed promise without actually indicating superconductivity. Again, quoting Weinstein:
“Paul is the leader in developing new techniques for making the superconductor material.”
Meng’s notes indicate that Chu’s laboratory initially considered coprecipitation (a method employed by the IBM-Zurich team) but quickly turned to simple solid-state reaction from powders,[216] hardly new or sophisticated. Weinstein continues:
“There is some hope that Chu will get a Nobel Prize out of this.”
I cannot refute this assertion; clearly some were indeed pushing aggressively for this outcome.[217] Continuing:
“If he does, it will be for discovering the role of pressure.”
While pressure can have an effect on the critical temperature, it is unclear in this context what is implied by the word “role.” Byars then paraphrases Weinstein going forward:
Before Chu’s discovery, pressure was not thought to have any effect on superconducting materials.
This assertion is somewhat difficult to reconcile with the fact that the first observations of the effects of pressure on the critical temperatures of superconductors were made by Sizoo and Onnes in 1925,[218] predating Paul Chu’s birth by about sixteen years.[219]
Sadly, the Chronicle’s remarkably low bar for technical accuracy was a hurdle few subsequent accounts of the YBCO discovery were able to clear.
Who, What, When, Where? Novel Superconductivity
While the spring of 1987 saw panel presentations by Chu and Wu at the Materials Research Society and American Physical Society meetings, the first written narrative of any substance appears to have originated with a workshop conducted in Berkeley, California in June of 1987 entitled “The Novel Mechanisms of Superconductivity Conference.” That narrative is Chu’s Novel Superconductivity, the apparent birthplace of the J-31 legend and the first major steps towards what will become an ever-evolving story.
Towards reinventing the impetus for (and, at times, the site of) the YBCO discovery, the page marked 584 states:
It was, therefore very natural [given the high pressure tests combined with the J-31 “instability”] for us at Houston and Alabama to try to synthesize mixed phase[220] A-Ba-Cu-O compounds with A=Y, Yb and Lu… On January 29, 1987, we detected in the mixed phase Y-Ba-Cu-O (YBCO) compounds a resistance (R) drop, starting at 93 K and completing at 80 K.
With the acceleration of the patent interference, Houston would be forced to temporarily abandon the 29 January date, only to reclaim it after the close of the evidence discovery period.
Who, What, When, Where? Robert Hazen’s The Breakthrough
Given the substantial press coverage of the discovery, it was only a matter of time before someone wrote a book covering the event. Unfortunately, I would often only find out about such works by stumbling across them in the bookstore.
Robert Hazen, a talented writer, very skilled scientist, and leader of one of the teams to isolate the pure-phase YBCO superconductor, tapped Chu as a primary resource for his previously-mentioned book entitled The Breakthrough: The Race for the Superconductor.[221] He writes in his acknowledgments (page 260):
Paul Chu, in particular, has taken hours of his time to detail the historic events leading up to the discovery of the 1-2-3 phase…[222] He has been candid about his successes and failure, and he has consistently taken more pleasure in praising his colleagues than talking about himself.
With all due respect, my preference is to be acknowledged for my ideas; a generic “thank you” for my “hard work” casts me as a bit player in Chu’s “loosely-based-on-real-events” tale.
Hazen next describes discussions with members of the Houston Lab, including coauthors on the original YBCO paper (Li Gao, Pei-Herng Hor, Zhi-Jun Huang, Ru-Ling Meng, and Ya-Qin Wang), some listed in the acknowledgments of that paper (Jeffrey Bechtold, the person running the “first tests” in the NOVA program to be revisited shortly), and others (Ken Forster, Theresa Lambert, and Simon Moss).
Much later in his acknowledgments, he mentions “thoughtful and constructive reviews” by M. K. Wu supposedly “detail[ing] the events surrounding the first synthesis of the 1-2-3 compound and… aspects of the cooperation between Houston and Huntsville.”
Two individuals of note were curiously absent from the acknowledgments, perhaps because Hazen had been led to believe they could offer no significant input to the story, namely C. J. Torng, the person who physically made the first sample, and myself. Another list where C. J. and I are curiously omitted will be discussed later.
When I am in a better mood (uncommon these days when doing anything connected with superconductivity, although I must confess that writing this narrative is mildly therapeutic), reading Hazen’s account can have me laughing almost to tears. No offense to Mr. Hazen; I envy his ability to write of technical matters and communicate them in a very readable way. It is simply unfortunate that the version of the story he was given by Chu would never appear again in any other forum.
I am tempted to quote huge portions of Hazen’s account, but I will try to maintain some measure of self-control. From page 43 of the chapter entitled “Pressure:”
While the Houston group was busy setting high-Tc records, Maw-Kuen Wu and the Alabama researchers at Chu’s bidding took another tack.
Hazen goes on to describe Chu’s “bold thinking” where, inspired by his high pressure results, he decides to achieve the same effect by substituting smaller atoms. However, Hazen soon concedes:
Even without the striking high-pressure results, the strontium-for-barium substitution was attempted by several other labs.
I wonder if the many papers by the aforementioned French chemists (who had made the strontium substitution years before) might have played a factor, too. After describing Houston’s successful “publicity effort” going into the New Year, Hazen states, “Chu’s high pressure experiments pointed the way to new superconductors.” Recall that, with the lanthanum-barium-copper-oxide samples always as Chu’s reference, there were no trivalent candidates larger than La3+ and no divalent candidates larger than Ba2+ (lest someone was up for working with radium or actinium). The only direction to go was smaller. Thus, Chu’s high pressure results were essentially pointing the way to the rest of the periodic table.
Chapter 3, “The Yttrium Breakthrough,” picks up with the start of the New Year (1987):
It was time for more bold thinking, but first there were a few clerical details to clear up.
Let it never been said that the YBCO saga is short on irony. After describing on page 41 that strontium had been an “obvious choice” to replace its next larger sibling barium, Hazen proceeds to offer an excellent description of the basic thinking no doubt going on at the same time in every other superconductor lab on the planet, concluding (page 49, my emphasis) with:
So lanthanum was the obvious next element to alter.
Hazen proceeds,
With the high-pressure data as a guide [pointing the way to the rest of the periodic table], Chu selected three possible substitution elements… He… targeted [among others] the obscure element yttrium… The search for higher Tc was the next project for the Alabama group…
Thus, in barium, strontium, lanthanum, and yttrium, Hazen has mapped out my Four Corners analogy. He continues (page 49, my emphases),
Chu and Wu agree to stick with the 2-1-4 composition by always mixing one unit of copper for every two units of the larger metal elements,
which is bizarre, given that 1) J-31 specifically violated this agreement and 2) on the previous page, we learned,
Paul Chu was convinced that the ultimate answer to high Tc was not going to be found in the 2-1-4 compounds… If there was a 70 K superconductor it wasn’t 2-1-4.
I cannot help but composite these last two quotes just to highlight the extent to which the story stretches the frontier of plausibility:
Paul Chu was convinced that the ultimate answer to high Tc was not going to be found in the 2-1-4 compounds… If there was a 70 K superconductor it wasn’t 2-1-4… [so] Chu and Wu agree to stick with the 2-1-4 composition by always mixing one unit of copper for every two units of the larger metal elements.
Clearly, Hazen faced an all but impossible task in translating his incoherent sources into a coherent story. He continues:
Three Alabama physicists – Wu, Ashburn, and Taiwan-born graduate student Chuan-Jue Torng – prepared the two-to-one mixtures with many different compositions.
Not too bad so far, but recall that almost all of the new compositions tested in Huntsville during this period, unlike the Houston samples, fell outside the scope of the 12 January 1987 patent application presumed to outline Chu’s “carefully planned substitution program.” Continuing (page 49),
They [the Huntsville team] substituted different proportions of rare earths for lanthanum along with calcium or strontium or lead for barium. Throughout the month [of January] samples were ground, baked, cooled, annealed, and tested for electrical conductivity at low pressure. Gradually, by trial and error, they were able to raise Tc. The results seemed exceptionally promising, with frequent hints of resistance drops and other anomalies at temperatures from 50 K up to an astonishing 100 K. Acting as an extension of the Houston lab, the Alabama researchers kept in close touch with Chu, advising him almost daily of their progress and heeding his advice.
The substitutions described are a rather poor match to what was actually made and tested in Huntsville during this period, but they happen to be completely covered by the “short list” of samples previously examined from Meng’s notebook page H476, samples made and tested in Houston.[223] That single page includes rare earths cerium, yttrium, and lutetium as well as calcium, strontium, and lead substitutions for barium. Apparently, there have been few inhibitions against relocating work from one city to another when it was convenient.
Concerning the “frequent hints… from 50 K up to an astonishing 100 K” in Huntsville, these simply did not happen, yet another case of events relocated from Houston (recall J-31). The migration of events from Houston to Huntsville should come as little surprise. Hazen’s book represents Chu’s one and only attempt, albeit indirect, to include meaningful details of the activities in Huntsville in the critical weeks leading up to the discovery. However, having no real knowledge of those events, his best alternative was to use the Houston activities as surrogates. He lays no claim, in Hazen’s book or elsewhere, to any of the real compositions, samples, and tests in Huntsville prior to 29 January.
The next paragraph in Breakthrough states that the aforementioned Houston Dean of Sciences, “upon learning of the startling new results,” urges Chu to file a patent, which he drafts, according to Hazen, on January 9th before returning to Washington.[224] The reader might note that, in actuality, we ran precisely three tests on exactly two unremarkable samples, La1.8Sr0.2CuO4 and La1.8Ca0.2CuO4 (nothing new), between our return from Houston and the arrival of our liquid helium on January 9th. Somehow one is to believe that yttrium samples were showing “startling results” even before they were tested, fabricated, had the necessary raw materials secured, or even had the process of securing the raw materials initiated.
A piece in Time magazine from a year earlier might have previewed this drift towards a timeline where YBCO results were supposedly achieved prior to the 12 January patent application (and certainly simplifying “reduction to practice” arguments). In the article, Michael Lemonick writes:[225]
The new substance [Y-Ba-Cu-O] showed so much promise that Chu filed a patent application on Jan. 12. That promise was soon fulfilled. At the end of the month, after subjecting their creation to a series of heat and chemical treatments, Wu and his assistants began chilling a bit of the compound, by dousing it with liquid nitrogen, and sending an electric current through it. To their amazement, the sample’s resistance began to drop sharply at a towering 93 K.
Back to Hazen’s story, he continues (page 50),
By the second week of January evidence for the near-100-K effect was growing.
While this was simply not happening in Huntsville, some hint to the origins of this story may lie in the rest of the paragraph, which concludes with a detailed description of the J-31 legend. Hazen never identifies the sample by name, but the many details (“La-Ba-Cu-oxide rich in barium,” “odd pinkish color on the outside,” “large Meissner effect starting at 100 K,” “effect disappeared the next day”) leave little doubt. Presumably Chu must have been evasive about the ratios even then, as Hazen notes them to be “supposedly of the 2-1-4,” which, as seen previously from Meng’s notebooks, was not the case.
The next paragraph describes the story of the undergraduate students purportedly making samples wrong (recall Robert Pool’s Science article) but makes no specific mention of any sample containing yttrium. The story notes that Chu responds by telling “Ru-Ling Meng, who had a special knack for the tricky synthesis, that she would have to get back to the grind.” I was quite moved to sarcasm in response to the “trickiness,” but Hazen takes the words out of my mouth. Jumping ahead briefly to page 147, Hazen’s words in reference to YBCO read,
Anyone with a furnace and the right chemicals could make the stuff.
And again on page 255,
High-temperature superconductors can be cooked up in high school chemistry class…
Resuming the story on page 51,
Time and again they [Alabama] glimpsed oddities in the electrical resistance suggestive of superconductivity. Transient effects near 100 K were becoming commonplace. Gradually, as evidence became stronger, the Alabama team narrowed its search to a promising composition in the yttrium-barium-copper-oxide system – approximately Y1.2Ba0.8CuO4… So confident was Wu and his colleagues of success that they abandoned the tedious and expensive liquid helium… and made their preliminary measurements in an open bottle of cheap, 77-K liquid nitrogen.
Riveting, except that Huntsville test results (included in their entirety with this narrative) show no such “commonplace transients;” there was no attempt in Huntsville to even obtain yttrium until the third week of January; no yttrium sample was made until the 28th; the very first composition was Y1.2Ba0.8CuO4; it was tested first in liquid helium; and it indicated stable and reproducible superconductivity starting around 93 K with the better samples reaching “zero” (within the precision of the apparatus) around 80 K.[226] Hazen’s next paragraph describes the discovery event on the 29th of January for which the reader is referred to Part I of this narrative for an accurate account.
Hazen’s fourth chapter (February 1987) begins with a supposed agreement by Houston and Huntsville to “stick to the basic composition: Y1.2Ba0.8CuO4.” Had that composition indeed been the product of weeks of zeroing in on the formula, such an agreement might have made sense. However, the opposite was true. No variants on the ratios had yet been tried. Thus, different ratios were the obvious first thing to do (see Meng’s Exhibit G[227]).
Page 59 describes the assembly of the original YBCO paper with this line:
The three University of Alabama scientists, Wu, Ashburn, and Torng, were listed first, while six Houston physicists followed. Paul Chu insisted that his name appear last, in spite of his central role.
While this makes for a good story, my specific recollections were very different, and I would suggest that Chu’s decision to be listed last was motivated by the fact that his only other option was to appear in the middle of the pack. After devoting eight pages (61-68) to the aforementioned “Ytterbium” controversy, Hazen’s narrative soon moves to his own personal involvement in isolating the pure-phase YBCO superconductor, an account that I can only presume to be largely accurate from that point forward.
In fairness to Dr. Hazen and having quoted and criticized so much of his story,[228] I will strongly encourage the reader to purchase the book. I think one will find it especially fascinating when considered in light of the additional information offered here.
Who, What, When, Where? NOVA
In hindsight, I suppose it was inevitable that the good people at WGBH Boston would dedicate a NOVA episode to high temperature superconductivity, and, paralleling my “discovery” of Hazen’s book, I learned about the existence of the program surfing channels. The episode ultimately earned an Emmy for first-time writer, producer, and director Linda Garmon. It makes no mention of the Huntsville group, reenacting the YBCO discovery as if it occurred in Houston. Chu plays himself. The individual performing the tests in the dramatically darkened lab is Jeffrey Bechtold. As a matter of habit, we in Huntsville generally kept the lights on, but then I am not one much for drama. My favorite line is how substituting yttrium for lanthanum was a “chemically bolder move” than substituting strontium for barium. With all due respect, having actually been there, all this dramatic verbiage teeters between humorous and nauseating. I have included here three short clips digitized from my VHS recording from so many years ago as well as a transcript of the segment encompassing these clips.
The scene opens with a “reenactment,” Chu standing before a wall-mounted periodic table with Hor, Meng, and J. Bechtold pretending to listen...
Narrator: In Chu’s lab back in Houston, they continued to vary the original recipe using the periodic table for guidance.
Chu (pointing to the alkali earths column): Now let’s look at this periodic table. As you move up, the elements are getting smaller and smaller.
Narrator: By now, they knew other labs were getting in on the act, also improving the Swiss compound, in one case, substituting the metal barium with the smaller atom strontium. So they planned a chemically bolder move, trading yttrium for lanthanum.
Chu (sitting in front of a periodic table, apparently speaking to an interviewer): In 1984, I had a dream. I got some superconductivity above 77 degrees in a sodium sulfide system, and the next day I called my group together. I told them about this dream, and they got all excited. They went to the libraries, looked up all the possible compounds and tested them all. Too bad. Not only did [sic.] they were not superconducting, they were not even metallic. Not only that, we also tested them under pressure. In fact, I should tell you, we squeezed hair, too, you know. Of course, no superconductivity.
Scene opens with someone (Meng?) putting a sample in a furnace. The scene transitions to a clip of someone handling a green pellet with tweezers, then transitions again to J. Bechtold taking hold of the one of the probes typically used for lowering a sample into a dewar for resistivity testing, and finally to Bechtold operating a computer with Chu looking over his shoulder at the screen.
Narrator: This willingness to test anything and everything was about to earn Chu’s lab a place in science history, for by adding yttrium, they produced a strange greenish mixture. Green usually indicates you got a useless insulator, not a supeconductor, but Chu still ordered tests, hoping some tiny portion of it might superconduct.
Bechtold: OK, here, Dr. Chu, I have the graph on file, and I’ll call it up and [unintelligible].
Narrator: The characteristic drop said it all. Something did superconduct at a record- breaking 93 degrees Kelvin, -292 Fahrenheit. It took nearly a century to go from 4 degrees Kelvin to 35. It took only months for Chu to raise that record to dramatic new heights.
Clips and transcript from NOVA's "Race for the Superconductor."[229]
The story captured on video, while brief, is roughly consistent with what will be presented by the University of Houston to the U. S. Patent and Trademark Office in the course of the interference with UAH (see the next section). Strangely however, it is a story that will have almost nothing in common with relatively detailed accounts Chu will will publish in a series of papers some eight to nine years later.
Who, What, When, Where? Science Magazine
Having made several references already to Robert Pool’s August 1988 article in Science magazine,[230] it seems prudent to include here a collection of additional relevant excerpts, especially those where Pool noted that Wu corroborated my story (a factor given high priority since the article had to pass muster with the publication’s attorneys). When asked for his general assessment, Wu responds, “The contribution from both sides was equal, in my view.” However, when asked about specifics, we find (my emphasis):
According to Wu's and Ashburn's accounts of the Alabama team's work, its efforts were essentially independent of Chu's direction and advice.
A comparison of the lab notebooks with each other and Chu’s January patent applications would seem to alleviate the need for any qualifiers. Next, Wu refutes Hazen’s account of the January 1987 activities in Huntsville (my emphasis),
Yet Wu and Ashburn say, the month was mostly spent looking at the strontium substitution and the yttrium success came on the first try. They were not seeing tantalizing hints of superconductivity at temperatures near 100 K, such as Chu was, and they were not getting day-to-day direction from Chu.
Finally, Wu explicitly maintains that Chu’s pressure work played no role in the discovery and confirms that there was no attempt in Huntsville to obtain yttrium until late in January (my emphases),
On the other hand, Wu and Ashburn say, Chu's high-pressure studies had nothing to do with their own decision to try yttrium -- it was due completely to Ashburn's back-of-homework calculations-and note they did not even try to get yttrium until late in January.
Wu goes on to corroborate my assertion that Chu had no knowledge of our work with yttrium prior to the discovery, concedes that yttrium was a natural candidate to be considered, and notes that it was never singled out [by Houston] from other possibilities ,as is evident from the Houston notebooks and January patent applications (my emphasis),
The Alabama team decided to look at yttrium for totally independent reasons, and Chu was not even aware that Wu's team was working with yttrium until the good-news phone call on 29 January, Wu and Ashburn say. All the earlier discussions of yttrium were basically irrelevant to the Alabama team, the two say, because yttrium was just one in a range of elements that were natural candidates for substitution into the existing superconductor formula and yttrium was never singled out from other possibilities.
Meanwhile, Chu in the article continues to maintain the significance of the pressure measurements, reduces the early January "tasking" to a conversation where “the yttrium substitution was talked about” (by "the," I assume he is referring to the failed yttrium samples made soon after in Houston), and then raises all manner of question about the degree to which the two groups were coordinated by pointing out,
If the University of Alabama in Huntsville group had not found the yttrium material, Chu says, the Houston group probably would have soon.
Who, What, When, Where? Patents and Perjury
The next accounts of note coming out of Houston followed in the early ‘90s with the patent interference case. As of this writing, I have what were originally four file boxes (successfully squeezed into three so that I will not mislead as to the actual volume of material). I suppose one could write yet another book on the case itself, but, in my opinion most woefully ignorant of intellectual property matters, much of the legal banter seemed only to serve the purpose of distraction and diversion. Out of what would be a stack of papers about as tall as I, one might extract, at most, a single medium-sized three ring binder of material of any real meaning.
In short, the story presented by Wu, C. J., and me to the patent court was largely the one I presented in Part I, that plus the stamp of the attorneys trying to ensure that we checked whatever legal boxes we must. To my knowledge, any declarations prepared by Chu (see Appendix C) or Hor were never filed. Meng, on the other hand and for reasons she will describe shortly, was tasked with telling what was perhaps the most unique of the Houston stories. Starting with the existing record, the contributions of the Huntsville team in the course of the YBCO discovery were simply erased and the remainder of the story, with its holes, was heavily patched. Because January 30th and 31st were spent performing tests inconveniently labeled as samples belonging to “Wu,”[231] those were among the results withheld (until I obtained copies within the last few years). Thus, by the Houston account, the discovery occurs on the 1st of February, a date Chu has never recognized in anything else he has written (feel free to browse the linked Meng documents from the original patent case, my “rebuttal,”[232] and Chu’s various accounts covered herein).
Having previously mentioned Ruling Meng’s 2006 “perjury” affidavit, I will cover that now. A copy has been linked for the reader’s review.[233] The document came about as a consequence of a lawsuit between Peiherng Hor and Chu, one that seemed to waffle between claims on the YBCO inventorship and subsequent claims on YBCO variants that instead employ the various lanthanide elements.
Meng’s affidavit includes several interesting parts. The very top of the page marked 3 yet again belabors the argument about who was the first to suggest “visiting Colorado” (see Part I for my Four Corners analogy). The next paragraph has her story of urging us to “try NASA” to find yttrium oxide (as noted previously, even before we had a chance to check the UAH stockrooms first). She indicates that, by checking with “NASA,” “this could speed up the process, because we do not have Y2O3 and it [sic.] if I placed the order the next day [January 5th], it would take two weeks for us to get our order.” Incidentally, even Hor’s concurrent affidavit[234] indicates that the Houston team was similarly inclined to check their stockroom first (page 3). I will return to this shortly. I will also later demonstrate that Meng’s suggestion of a two-week turnout on the order is indeed false.
This is followed by her own version of the J-31 story, which, apparently with so many retellings by Chu, she too had come to believe to be true. However, for whatever reason, Meng assigns the “transition up to 90 K” to the sample identified as J-2 instead. J-2 first appears in her notes[235] on the page stamped H490 dated 5 December 1986. Page H490 is also the last page I have been able to locate that references sample J-2. Her affidavit mistakenly identifies J-2’s composition as (La0.4Ba0.6)2CuO4, but her notes show it as (La0.6Ba0.4)2CuO4. Recall that by building his story on J-31, Chu selected one of the few samples with the right ratios of the first two elements (ratios that matched that of the YBCO discovery material). However, J-31 had twice the copper as did the composition responsible for the YBCO discovery. Meng, on the other hand, fixes that little problem by going back 5-1/2 weeks to one of the very first copper oxide samples made in Houston, one that matched the ratios perfectly, including the copper, thus being only an yttrium-for-lanthanum substitution away from the pivotal January 29th formula. So close and yet so far.
According to Meng, sample J-2 was discovered, after the fact, as containing the lanthanum equivalent of the pure-phase YBCO superconductor (aka, the “123 structure”). Meng states that the powder X-ray diffraction data showed “almost pure 123 structure,” although even in the most unrealistically ideal of circumstances, it could not have had more than 50% by volume of the 123 structure given metals in the ratios 1.2:0.8:1.0. The X-ray diffraction data she references appears on a chart apparently taken from the same presentation as the “Clear Evidence” chart. A copy of the chart is shown here:
X-ray diffraction data attributed by Ruling Meng to Houston sample J-2.
Oddly, what Meng attributes to sample J-2 is clearly marked as belonging to sample J-36 (of composition (La0.5Ba0.5)5Cu5O5(3-3y) per Meng’s page stamped H473).[236] The origins of this confusion between J-2 and J-36 are likely traceable to Chu’s aforementioned 1996 “High Temperature Superconductivity” paper.[237] [238] On the page marked 810 in that discourse, the caption under the x-ray diffraction pattern marked J-36 states that the pattern belongs to the same LBCO sample whose magnetic susceptibility tests are depicted on the paper’s immediately preceding page, a sample identified there as J-31 (only the “-31” is visible in this particular graphic).
The chemistry of La1+xBa2-xCu3O7-y (a formula that effectively covers J-36 and the Er-Rakho et al. material) is vastly more complicated than YBa2Cu3O7-y, and there is substantial literature devoted to the topic. See, for example, the work of Shu Li and Martha Greenblatt.[239] First, Li and Greenblatt, like many others, describe preparation of the high Tc (~90 K) La-Ba-Cu-O phase as “difficult” (as compared to its YBCO cousin). Second, La1+xBa2-xCu3O7-y spans a series of related structures with varying x and y (most of which are not superconducting), all producing nearly identical X-ray diffraction patterns that are very difficult to distinguish without the most precise measurements and analysis.[240] Third, superconductivity only occurs when x is in the range of 0 to 0.5 and y is small, the latter condition only achievable by annealing in oxygen in the range of 300 to 600 °C (Li and Greenblatt preferred 450 to 500 °C). Thus, early in the analysis of this family of materials, superconductivity was most often observed below 77 K, with critical temperatures approaching 90 K only with more precise adjustments in x and y as achieved by judicious coordination of the starting composition and multi-stage processing. To this I would add that Chandehari and Brass specifically describe the 90 K LBCO superconducting phase as only forming in an oxygen atmosphere and decomposing when heated in an inert [oxygen-free] atmosphere.[241] [242]
Whatever the case with J-36, Meng’s notes (again, page stamped H473) indicate that its resistivity testing showed only a broad transition between 30 K and 8 K. Thus, whatever J-36 is, neither the x-ray diffraction patterns nor the resistivity tests provide any conclusive evidence for the presence of a 90 K superconductor. In my estimation, J-36 is at best a reproduction of the La3Ba3Cu6O14 material made first by Er-Rakho et al. in 1981.
With regard to the “90 K transition,” she cites “Reference 3,” which is, in fact, the undated J-31 (not J-2) chart discussed ad nauseam earlier (see the “Clear Evidence” chart noting “Reference 3” penned beneath the “Clear Evidence” heading). In Meng’s defense, the top left inset from the first page of Chu’s “Superconductivity Above 90 K” paper obscures the “J-31” designator (or at least the “-31” part).
In any case, by combining the composition of J-2 from early December with the dubious J-31 magnetic tests and the powder X-ray diffraction from J-36 (the latter two apparently from mid-January), Meng has brought together, after the fact, the desired samples ratios, a curious but hopelessly suspicious magnetic susceptibility test, and ambiguous evidence for the 90 K lanthanum “123” crystal structure determined only after the fact. If only the three of these had, in reality, been the same sample, they might have made for a credible story.[243]
If I had the energy right now, I would probably investigate the powder X-ray diffraction chart to see just how pure it is, but, quite honestly, I am very tired, having suffered with a persistent cough for several months now combined with arising around four o’clock every morning to put in a couple more hours of writing on this accursed book. Whenever I dig, the story only becomes even more convoluted. I did briefly sift through Hazen’s book hoping to latch a good pattern on the pure-phase 90 K superconductor, only to find on page 57 an X-ray diffraction chart sent from Chu to Hazen some time in February. Unfortunately, the caption notes, “The pattern is further complicated by systematic errors that were introduced to hinder unauthorized analysis… Geophysical Laboratory scientists were sent this doctored chart by mistake.” Perhaps there really is no bottom to this pit.
Meng goes on to state (page 3) that on the 13th of January, twelve days after the order was placed (by her reckoning), the yttrium oxide arrives. This timeline is especially intriguing since Chu’s later papers, which will soon be explored more closely, actually place the order some time in mid-January, with the shipment arriving on or just prior to the 29th. Perhaps the best way to put the matter to rest would be to actually consult the documentation. Fortunately, the ongoing (as of this writing) Hor-Chu case (see Appendix D) has kindly obliged. I have included here document 82-39 from Case 4:08-cv-03584, the requisition where yttrium oxide among other reagents was ordered in Houston.
Standing Account Requisition Form, Chemistry Department, University of Houston, 12 January 1987 (sic.).
One will note underlined with emphasis “Next Day” in the middle of the form. Presumably Meng must have inferred from this a January 13th arrival (making it mysterious that she seems to continually suggest a two week turnaround, although the two week argument is later refined in the Hor-Chu case – see Appendix D). The actual “received date” appears to be the 14th, as indicated in the “Date Rcd” column. In any case, it is consistent with the short-lived mid-January efforts with yttrium (insulating failures YB-1 and YS-1) whose existence is routinely ignored by the Houston team members. Note also that three (cerium, lutetium, and yttrium) of the four rare earth oxides on the requisition are also covered in the mid-January series of tests documented on page H476 of Meng’s notebooks.[244]
Somewhere between the passing reference to yttrium around the New Year and the 29th of January by Meng’s account, “stuff” presumably happens in Huntsville (about which we get few details), after which she now suddenly after many years has a much clearer recollection of the “good news” phone call of 29 January. Page 4 eventually folds into a description of how Meng consented to lying in defense of Chu during the patent proceedings. Ironically, when I first penned this part of my manuscript, I largely glossed over her perjury confession, but I have since decided it might be worthy of some review.
I will first note that Meng’s admission seems to conclude a section on the question of YBCO inventorship, specifically one that, in her mind, revolves entirely around who first uttered “yttrium.” Without belaboring the details, my main point here is that Meng’s prior credibility (upon which rested all of the details of Houston’s claim on YBCO) is self-discredited. Here are some excerpts (grammar and punctuation errors are from the original, emphases are mine):
[Houston attorney] Charles Cox called me and asked me again. that whether I remember Dr. Chu had called and directed me to replace La by Y. I did not answer and he said if you would not identify Dr. Chu told to you about his idea about the substitution of La by Y. Then the University of Houston would lose the patent to University of Alabama. I immediately said no this was our (UH) idea and they learned from us. Then Charles said then we need to identify Dr. Chu, he represents UH. I knew, if I say yes! I was lying. I asked Cox. whether I had to go to court? He said, “No, no, you would not go to court” I knew this patent should be ours (UH). I do every thing to help UH earn the patent. Therefore, I said, “maybe Dr. Chu had called me and talk about the substitution.”
One day, unexpectedly, Charles brought me the declaration writing by him or Chu and asked me to sign. All the detail and date was written and by Cox or Chu all I did was signing my signature. After that Dr. Chu thanked me for helping in the critical time.
In 1993, I was asking to make a deposition for U. H against the Uni. Of Alabama. I do not have any choice but continued to lie by testifying Dr. Chu had called and told me about the substitution of La with Y. I feel very uncomfortable about this. But I think I did it for UH. Beside this all of my other statements in the deposition are truth.
An examination of Meng’s deposition (linked here[245]) suggests that her final assertion is tenuous, at best. I will examine some of the highlights here (for other details, the reader might peruse my older analysis of her testimony[246]).
On page 11, Meng is unable to recall the late January testing in Houston on Huntsville samples. On page 28, she describes how Chu directed her to replace lanthanum with yttrium in their La-Ba-Cu-O samples. In attempting to explain why yttrium should be paired with barium instead of strontium, she states (my emphasis):
Obviously only replace lanthanum so the formulas definitely should be yttrium, barium, copper, oxide…
Concerning the “obviousness” of the combination, the reader might review page H476 from Meng’s notes where her first listed yttrium sample is yttrium-strontium-copper-oxide (YS-1), not yttrium-barium-copper-oxide (YB-1).[247] Clearly what is not obvious is why someone would do first what is “obviously” not the right thing to do.
On pages 34-36, she indicates that the Huntsville-made La-Sr-Cu-O samples were “too poor” for publication because of wide transitions. One can only imagine how it is that wide transitions (like those of the original Nobel-winning Bednorz-Müller paper) are deemed unworthy of publication while irreproducible and dubious tests like those on J-31 or Sample #1b (Appendix A) seem to routinely find their way into print. Continuing through page 38, Meng describes how the implied incompetence of the Huntsville team with La-Sr-Cu-O served to only delay her work with yttrium and thus, by inference, delay the YBCO discovery.
One pages 42-43, counsel asks Meng about the “concept of the substitution of yttrium for lanthanum during that [New Year’s trip] discussion.” Meng notes that Chu was not present. She cannot recall who first mentioned yttrium in Wu’s presence except that she specifically recalls that it was a member of the Houston group. She furthermore claims to recall that Wu, among a group of less senior people, “definitely [does] not” contribute to the discussion. Kelber asks if she recalls anything else discussed (presumably probing for something on the elemental ratios or processing) to which she responds, “Not really.” I would note here that Meng repeatedly confuses the word “ratios” (in questions regarding the relative amounts of the elements) with radii (sizes of the atoms/ions); thus, counsel was never able to establish her perception of how the ratios in the critical YBCO composition were achieved.
On pages 56-58 in a discussion on the five-week period from Christmas to the 28th of January, Meng describes the making and testing of 85 samples as “a lot,” stating that it often required working “day and night.” At such a pace, it would only have required Meng thirty years to test one each of the elemental combinations covered in Chu’s early January patent application (as of this writing – 2015 – she could almost be done by now[248]), all of course assuming that only one set of ratios and one set of processing conditions were examined for each combination.[249]
On pages 78-79, she describes the yttrium oxide order being placed on 12 January. She then indicates that it did not arrive until two weeks later (again, the supposed “two-week turnaround”), supposedly prompting what she indicates as her first work with yttrium on the 29th of January (again ignoring the earlier mid-January YS-1 and YB-1 samples). She describes the arrival of the chemicals as the “number one reason” for starting at that time, mysterious in light of the statement in her affidavit (page 3) that the yttrium oxide arrived on the 13th. Despite having morphed what is clearly a next-day delivery into a two-week delay, she seems to be working to stretch it even further to cover the entire month of January; by her affidavit we have yttrium oxide arriving on the 13th of January after what she describes as a two-week delay (page 3) following an unspecified order date, while her deposition describes placing the order on 12 January with the yttrium oxide arriving some time around the 29th, again two weeks later. Unfortunately, the only consistent part of her story (a two-week delay) is the part most wrong (though convenient for justifying other aspects of her story). I would again remind the reader of the copy of the “Next Day” requisition examined earlier, consistent with the only two precise dates given by Meng albeit in separate accounts, concerning the order and receipt of yttrium oxide.
On page 79, she describes the 29 January phone call from Wu to Chu concerning the “higher transition about 77 something” and then describes Wu coming to Houston to confirm superconductivity. On pages 82-83, counsel asks Meng, “Did you in fact test the sample that Dr. Wu brought?” She responds, “I remember we did” but does “not exactly” recall the results. She starts to describe her “impression” of those results when interrupted by her attorney. She confirms that Houston had records of those tests, but then later qualifies “I think we measure his sample.” By the end of page 83, she is “not quite sure” they had records of the testing. On page 84, copies of the test results are requested; they did not make an appearance until about fifteen years later in the ongoing (as of this writing) Hor-Chu case (see Appendix D for more).[250]
On page 90-91, Meng attempts to explain why the YBCO sample with Y:Ba in the critical 1.2:0.8 ratio (YB-102) was examined first despite not being listed so in her notes. She responds, “That’s the best sample at that time we had.” How does one know which is the best before testing it? Unless, of course, her work was taking its cues from the Huntsville team.
One pages 99-101, Meng attempts to explain her sudden work in early February on La-Hg-Cu-O. I would remind the reader of the importance of a La-Hg-Cu-O sample tested in Huntsville in late January (see Part I) and the sudden appearance of mercury in the February 6th Houston patent application. Meng’s explanation is that the composition was based upon “some kind of principle… some kind of rule to follow,” but she offers no elaboration.[251] She describes mercury as having five possible valence states (not true[252]), that its valence might match that of copper (yes, but irrelevant since the compositions indicate that mercury was assumed to replace the alkali earths, not copper), and that its atomic radius is much greater than barium (also not true).
The next chapter of this manuscript will review the hectic events of the last two days of January 1987 as described by Chu. Meng’s inability to recall those events will raise some questions with interesting implications.
Beyond the fact that Meng’s affidavit may understate the scope of the falsehoods, it can at least be argued that most of her affidavit is true (starting with the admission of lying). My guess is that almost everyone in Huntsville and Houston who claims to have a recollection of “conceiving of the idea to try yttrium” (whatever that specifically means) is probably telling the truth. As I have already established, that can still leave one far from a 90 K superconductor. In any case, aside from the all-too-perfect J-2/J-31/J-36 amalgamation, Meng’s affidavit marks a substantial improvement over her past testimony.
In describing her experience, she writes (page marked 5):
However, no matter what the reason, I should not have lied. The guilty feeling haunted me for about 20 years. Due to my health situation. I am planning to retire soon, I do not want to carry this guilty feeling to my retirement life. I want to have a peaceful mind. After a long mind [sic.] struggling.
In Meng’s defense, she, like me, was just another pawn in an insane game. Rest assured, upon obtaining a copy of her affidavit, my first instinct was to push for criminal charges against her. If she might happen to read this, she can take it as my commitment to her to not do so. I spoke to Hor some time back, and he described when she came into his office (an event she recounts briefly in her affidavit). Hor informed me that she was in tears from the guilt over her earlier testimony. Note that she was the only one deposed during the ordeal, pressed to try to fill in the missing details of a fragile story she had been tasked to defend.
Reality tends to lend itself to details. Fiction, on the other hand, not so much. I am not sure why her updated story continues to be so confused, but I do commend her for her courage to admit some of what she had done.
One can only imagine how the patent case might have played out had the examiners the opportunity to review the missing test results in Houston (“Wu’s #2 1-30-87,”[253] for example), Chu’s desk calendar[254] (“Observed Tc>77K” on January 29th), and his mid-’90s papers establishing the correct composition, date, and location of the discovery, which leads us to the next section.
Who, What, When, Where? Chu’s Mid-’90s Papers
What I usually call “Chu’s mid-’90s papers,” a series from 1996 and 1997, some of which have already been briefly introduced, together provide Chu’s most detailed first-hand account of the discovery. The reader should be prepared for yet another brand new story, although this one is, oddly enough, among Chu’s most accurate.
In addition to the two articles linked previously,[255] [256] a third similar account appears in an IEEE Transaction.[257] The reader is encouraged to compare the three. The “History of” version seems to provide the most details, so I will use it as the baseline. The page marked 808, just across from the dramatic chart on sample J-31, reads as follows (my emphases):
After I finished the preliminary draft of the 57 K LaB-214 paper and was just about to write the 90 K LBCO paper, I received an exciting call from Maw-Kuen from UAH at about 5 p.m., on January 29, 1987. He informed me that he and his students, Jim Ashburn and C. J. Torng, had just observed a reversible sharp R-drop starting at above 90 K and finishing at about 77 K in two of their samples. All of us were ecstatic, since stable and reversible superconductivity might finally have been achieved provided a Meissner effect could be detected. Right before he called me, Maw-Kuen had also phoned Peiherng [Hor] about their exciting observation. Without divulging information about the elements of their samples, Maw-Kuen told Peiherng, “We just did what we discussed previously (in Houston in early January).” Peiherng, Ruling, and I reviewed all our previous data and decided to make a few samples containing the newly arrived Y and Yb oxides. Unfortunately, we failed to see any stable superconductivity at 90 K. Maw-Kuen and Jim arrived at Houston the next morning on January 30 with their samples which had a nominal formula of Y1.2Ba0.8CuO4 (YBCO). We subjected them to a thorough battery of tests. Indeed, R [resistivity] decreased rapidly, starting at ~93 K and reached zero at ~80 K, as was first observed at Huntsville by Maw-Kuen and his students. By the end of the day, we completed the magnetic measurements. A distinct diamagnetic shift characteristic of a superconducting transition started at ~91 K. The results are shown in Figs. 8 and 9. The expected downward shift of Tc toward lower temperature in the presence of a magnetic field was also observed. Following the recipe of Maw-Kuen, several samples were also made in Houston and tested on January 30. Most showed the 90 K superconductivity. The long-sought-after stable and reproducible superconductivity above the liquid nitrogen boiling point of 77 K had been discovered.
I will note that the “90 K LBCO paper” presumably concerns the J-31 “results.” Thankfully, it was never written. This is perhaps as good a point as any to interject that, in my prodigious collection of documents, I have yet to find one where Chu clearly states how the J-31 results actually influenced the direction of activities in Huntsville.
Most significantly, though, with these somewhat more recent versions added to the list of the more prominent accounts examined so far, the story has almost come full circle, having followed this circuitous path:
- Hazen account: January 29th discovery in Huntsville, Chu’s micromanagement
- NOVA: January 29th(?) discovery in Houston, no mention of Huntsville
- Patent Case: February 1st discovery in Houston
- Mid-’90s Papers: January 29th discovery in Huntsville, no micromanagement
Given that the first (Hazen’s book) and the last (Chu’s papers) here seem to have the date and the city correct, a comparison of at least those two seems warranted in the off-chance they can be reconciled.
While Hazen describes large numbers of yttrium samples made over the course of January (the “task” we had been “assigned”), gradually zeroing in on the right composition, with Chu seemingly micromanaging every facet of our work as “an extension of the Houston lab,” I recall no such micromanagement. Our notebooks clearly discredit Hazen’s story, and Chu has never made any mention of or laid any claim to any of the numerous failures made in Huntsville prior to the production of Y1.2Ba0.8CuO4 on the 28th (unless his patent claim on mercury is to be included).
By Chu’s account in his mid-’90s papers, he “thinks of yttrium” (along with everyone else in the superconductor community at the time and at least half of the onlookers), “narrows” the range of composition down to no less than tens of thousands of possibilities, fails with yttrium (apparently twice, once sometime between the 12th and 18th by Meng’s notebooks and, by these later papers, again on the 29th, both times just moments after what appear to be two separate arrivals of yttrium oxide shipments). During the stretch of time between the “bold thinking” and January 29th, Chu gives not a single mention of anything going on in Huntsville, not even a passing reference to the crescendo of excitement described by Hazen (and largely attributed to Chu). Back in Houston, we get only the J-31 tale, which I think I have sufficiently pummeled.
Instead, Chu offers
Without divulging information about the elements of their samples…
With all due respect, this sounds like the daily status reports were long overdue. Otherwise, surely we could not have hoped to set Houston back by more than a day or two by withholding the information (remember Hazen’s frequent use of the word “gradually”).
Then Chu has Maw-Kuen telling Peiherng,
We just did what we discussed previously (in Houston in early January),
suggesting that the daily status reports were about that far overdue.
Concerning “early January discussions,” by Hazen’s account (and he gives many surrounding details), Chu quickly drafts his first patent application on January 9th and polishes it in a “marathon weekend phone conversation” on the 10th (page 50). On the other hand, Pool’s Science article records Chu as saying that Wu was (somehow) shown the patent application (remember 26,775) during our New Year’s trip to Houston that ended on the 4th (page marked 657).[258] Chu tells Pool that, during our trip, the “yttrium substitution was talked about” (not quite tasking).
If what was discussed “in Houston in early January” was mutually understood, then surely after all of the issues with irreproducible results in the preceding weeks (e.g., J-31), Houston would immediately seek to reproduce the Huntsville results, and if those discussions indeed included sufficient information to achieve the Huntsville results, then why did Houston, by Chu’s account, completely fail yet again (recalling Houston’s unacknowledged mid-January yttrium failures,[259] somehow made before yttrium was even available in the Houston lab) to make an yttrium superconductor on 29 January?
By Hazen’s account, 29 January marked only the finishing touches in zeroing in on the optimal sample. Surely had we been in daily contact with Houston, they could have produced something on that day showing at least the faintest signs of 90 K superconductivity.
It is similarly notable that, by Chu’s account, the USPTO decision in the Houston-Huntsville interference – that the UAH team fabricated the nonsensical sub-oxide Y1.2Ba0.8CuO1 (oxygen ratio outside of the scope of the patent application but impossible given the processing conditions) – was erroneous; Chu specifically identifies the Huntsville composition as Y1.2Ba0.8CuO4. (oxygen ratio much nearer what the processing conditions mandated and within the scope of the patent).
I will speculate here concerning Wu’s statements. My recollection was that Wu did mention yttrium in the phone call to either Chu or Hor. However, I do not specifically remember any mention of barium or of the ratios. Recall that our focus had been almost exclusively on strontium over its larger cousin, and Wu certainly knew that Houston, were they to guess barium, would not possibly guess the ratios. So, Wu might have indeed been cautiously cryptic before making a final decision to turn to Houston for the magnetic measurements. I would furthermore suggest that Chu’s assertion that Wu said something about doing what was “discussed previously” is probably true, given what I now understand of the Wu-Chu relationship at that time. Note carefully, however, that I am saying that it is probably true that Wu said it, not that his statement itself was true.[260] At that point in time, we had no way to know that the higher barium ratio was actually critical to the result. Also, recall that Wu’s knowledge of what I had done to formulate Y1.2Ba0.8CuO4 was negligible. From his perspective (like Chu’s), Y1.2Ba0.8CuO4 seems to have almost magically appeared.
To the above excerpt, I would add an additional detail that appeared in Chu’s “Decade of” paper, more firmly narrowing the time of what Chu suggests are the first tests in Houston on samples containing yttrium:[261]
Without knowing the elements used in their samples, we tested a few samples, with La replaced by the newly arrived Y and Yb oxides. We failed to detect 90 K superconductivity in these samples the night before their arrival.
Of course, Meng’s notes already indicated tests on yttrium-containing samples in mid-January (recall my lengthy previous discussions of “YB-1”). My speculation here is that Chu’s mistaken[262] recollection of yttrium and ytterbium sample tests on 29 January was prompted by incorrectly dated pages from Meng’s notebook – Exhibits G and H from the original patent case.[263] Exhibit G was actually written on the 30th of January upon our arrival in Houston, as I specifically remember being present (let me emphasize here that this is one of the very few significant aspects of this story that I ask the reader to accept on the basis of my testimony alone). I would suggest that the original page was probably undated, and the “29 January 1986” date (obviously 1987 was intended) was probably added at the same time “29-” was prepended to the “30 January” date[264] on what appears by all accounts to be the next page in Meng’s notes – the first page of Meng’s Exhibit H (marked H52).[265] Concerning the possibility of originally undated pages, particularly during this time period, the reader may examine pages H52, H53, H54, H55, H56, H57, H58, H59, and H60 of Exhibit H. Regarding the potential for manipulations of the dates in the Meng notebooks, the reader should revisit my comparison in Part I of the various versions of Exhibit A as attached to Meng’s 1990 and 1993 declarations.[266]
Concerning the contents of Exhibit G, the page starts with a series of YBCO samples with varying amounts of barium substituted for yttrium. Recall that in Huntsville I skipped the “straight substitution” low-barium Y1.8Ba0.2CuO4 composition like Houston’s unacknowledged mid-January YB-1 sample,[267] going straight to high levels of barium. Thus, when we arrived in Houston, it was only logical to formulate a series of bracketing compositions to check the possibility of further optimizing the Y:Ba ratio. The second series replaces yttrium with lutetium (same valence, similar ionic size, and nonmagnetic), not ytterbium (which does finally appear several pages into Exhibit H at around the time the previously-discussed ytterbium “strategy” is formulated;[268] recall also the small letters “b” “test-written” in some of the yttrium compositions early in Exhibit H). Next is scandium (included over my objections that the ion was too close to copper in size to work, but those who use atomic radii instead might conclude otherwise). Next are several series with post-transition metal lead, prompted by the observations with mercury samples just days before in Huntsville (recall also mercury’s curiously-timed addition to the Houston patent applications).
For purposes of the analysis, let us instead momentarily accept the 29 January date in Meng’s notes as being in support of Chu’s account, that the samples listed in Exhibit G were indeed formulated in Houston without details on the Huntsville composition. Where does this lead us?
First of all, we have from Chu what appears to be yet another round of yttrium failures in Houston (even though we have been expected to believe that the mere mention of yttrium[269] around the New Year was sufficient to trigger the discovery in Huntsville), and this set of failures occurring, by Chu’s description, at a point in time where clearly the Huntsville team possesses the knowledge by which the “long-sought-after stable and reproducible superconductivity above the liquid nitrogen boiling point of 77 K” is achieved while Houston is still floundering. This would suggest that the Patent and Trademark Office has stretched the definition of inventor to unprecedented realms where the invention can actually precede the inventor’s sufficient knowledge to actually achieve his invention. If there is any doubt that there was indeed a prior mid-January series of samples with yttrium (and lutetium)[270] that followed the first(?) arrival of those oxides, the reader should note on the page marked H55 and those that follow[271] that Meng suddenly switches to three digit numbers on her sample identifiers to distinguish these series from the earlier yttrium and lutetium samples. Note on page H55 where she initially labels the first lutetium-barium-copper-oxide sample LuB-2 (having used “LuB-1” earlier in the month[272]). Then the “2” is scratched out and replaced with “102.” Next, “10” is squeezed after the dashes in LuB-3 and LuB-4 to make them LuB-103 and LuB-104. Having recognized the need for identifiers indicative of a new series of attempts with lutetium and yttrium, the compositions that follow, beginning with yttrium-barium-copper-oxide samples are then recorded with the three-digit identifiers (the first identified by YB-101 now instead of YB-1). Meng even references the otherwise unacknowledged YB-1 sample (along with the raw material weights that perfectly reproduce the “straight substitution” Y1.8Ba0.2CuO4 composition of that prior failure) in the margin of the page marked H56.[273]
Secondly, Chu’s account here indicates that these samples were formulated, fabricated, and tested starting sometime after 5 p.m. and completed that same night. Let us allow for the possibility that the Houston team pulled an “all-nighter” (such were admittedly not uncommon during that period), stretching the “night” into the wee morning hours. I will be generous and give them until sunrise (about 7:15 a.m. in Houston for that time of year). The reader is now encouraged to review Meng’s lab notebooks[274] and examine the total processing times she customarily used for this class of materials. Even though most samples appear to receive a minimum of 24 hours, with several rounds of heating over multiple days being very common, I will grant them the very shortest times I could find (in only a very few samples) – 12 hours.[275] This would leave Meng and her colleagues perhaps an hour to calculate and weigh out raw materials for what appears to be a rather long list of compositions, grind powders, press pellets, and load them into the furnace. After this, the team presumably sits around the lab for 12 hours (had they gone home, I would assume that Chu would have described the tests as occurring the next morning, and the window is insufficient to stagger the processing and testing), and then tests multiple samples in the remaining hour before the cock crows.
Third, having mentioned the calculation of the raw material weights, an examination of Meng’s record of this process is instructive. To find the weight calculations for at least 17 of the 25 of the compositions of Exhibit G, one must turn several pages into Exhibit H (samples list below in the order they appear in the Meng notes):
- Page marked H55:
- LuB-102, (Lu0.6Ba0.4)2CuO4 – Same as #6 from Exhibit G
- LuB-103, (Lu0.4Ba0.6)2CuO4 – Same as #7 from Exhibit G
- LuB-104, (Lu0.2Ba0.8)2CuO4 – Same as #8 from Exhibit G
- YB-101, (Y0.8Ba0.2)2CuO4 – Same as #1 from Exhibit G
- Page marked H56:
- YB-102, (Y0.6Ba0.4)2CuO4 – Same as #2 from Exhibit G
- YB-103, (Y0.4Ba0.6)2CuO4 – Same as #3 from Exhibit G
- YB-104, (Y0.2Ba0.8)2CuO4 – Same as #4 from Exhibit G
- YP-101, (Y0.8Pb0.2)2CuO4 – Same as #14 from Exhibit G
- Page marked H57:
- YB-105, (Y0.3Ba0.7)2CuO4 – Same as #26 from Exhibit G
- Two Yb-Ba-Cu-O compositions that do not appear in Exhibit G[276]
- Page marked H58:
- A third Yb-Ba-Cu-O composition that does not appear in Exhibit G
- Additional Y-Ba-Cu-O compositions that do not appear in Exhibit G
- Page marked H59
- LuB-101, (Lu0.8Ba0.2)2CuO4 – Same as #5 from Exhibit G
- YP-102, (Y0.6Pb0.4)2CuO4 – Same as #15 from Exhibit G
- YP-103, (Y0.4Pb0.6)2CuO4 – Same as #16 from Exhibit G
- YP-104, (Y0.2Pb0.8)2CuO4 – Same as #17 from Exhibit G
- Page marked H60:
- LuP-101, (Lu0.8Pb0.2)2CuO4 – Same as #18 from Exhibit G
- LuP-102, (Lu0.6Pb0.4)2CuO4 – Same as #19 from Exhibit G
- LuP-103, (Lu0.4Pb0.6)2CuO4 – Same as #20 from Exhibit G
- LuP-104, (Lu0.2Pb0.8)2CuO4 – Same as #21 from Exhibit G
Meng’s 1993 declaration[277] specifically associates the raw material weights in Exhibit H with the compositions listed on Exhibit G (supposedly formulated on 29 January independently of the Huntsville team’s activities), stating (paragraph 19 on page marked 9), “The amounts of reagents required for the production of the Y-Ba-Cu-O compositions as shown in Exhibit G are shown in Exhibit H.” Unfortunately for Chu’s timeline, between Exhibit G and the pages of Exhibit H listing the reagent amounts are the first three pages of Exhibit H (pages H51 to H53). These pages list the three-digit precise compositions I rederived in Part I using equations I have always maintained as the means by which the original critical Y1.2Ba0.8CuO4 composition was achieved. Barring some as yet unreported new elements to the story of when, how, and why I might have otherwise communicated these compositions to Houston late on the 29th,[278] I was in fact present in Houston when they were formulated and I was the one who formulated them (as previously demonstrated). Of course, Wu and I did not arrive until the 30th, as confirmed in Chu’s papers. Thus, there was no 29 January marathon of failed YBCO samples tested the night before the arrival of the Huntsville team since the corresponding reagent amounts appear in Meng’s notebooks after the unusual 3-digit precise compositions that I created on the 30th.
Fourth, one has to wonder why, after what Chu describes as the phone call from Huntsville reporting stable, reproducible signs of superconductivity around 90 K and knowing that the next few days would be grueling, he would, with no supposed knowledge of what was made in Huntsville, subject his team to an all-nighter on what would be by his account a long list of totally new and untested formulas.
Fifth, Meng’s declaration specifically identifies among the samples traceable to Exhibits G and H one labeled YB-102 (of the Y1.2Ba0.8CuO4 composition). In paragraph 20 (pages marked 9-10), she describes the processing of the sample at 1000 °C, a temperature more than sufficient to yield a substantial volume fraction of the 90 K superconducting phase and thus consistent with her tracing the progress of YB-102 through paragraph 21 (page marked 10) where she describes a portion sintered only 20 minutes[279] as being tested on 1 February and found to be superconducting starting at 94 K with zero resistance reached at 89 K. I have included for the reader a copy of Exhibit I referenced by Meng as showing the raw data from the first tests on sample YB-102.[280] The charts are all clearly dated “2-1-87” and plainly show sharp decreases in resistivity characteristic of superconductivity starting in the 93-94 K range and completing between 80 and 90 K (all as indicated by the markings). In contrast, Chu’s account indicates that this sample (unless there were other undocumented yttrium samples made that same action-packed evening) was tested late on the 29th and showed absolutely no evidence of 90 K superconductivity, leaving us to conclude that the sample experienced some kind of bizarre reverse instability, transitioning from no traces of superconductivity on the 29th to 24% diamagnetism on February 1st. The precise 24% figure appears in Meng’s declaration, paragraph 21, associated with February 5th, and by Chu’s hand on page 909 of the original YBCO paper (received by PRL February 6th and revised February 8th).[281] Of course, from Chu’s mid-’90s papers here, we know that the published results were based directly upon what he calls “Wu’s formula” and not on some effort in Houston independent of the Huntsville discovery as described by Meng.
Sixth, if we are to buy Chu’s story that these samples were actually made and tested in Houston without knowledge of what was made in Huntsville (and Meng’s patent testimony, while in conflict with Chu’s account, similarly attempts to disconnect the Houston activities from any prompting by Huntsville), then one must believe that Houston coincidentally tested the same composition made with the same processing temperature on the same date. While Meng’s notes showed a consistent history of altering only a single variable at a time when going from sample to sample,[282] Exhibit G marks a substantial shift from this pattern. Recall that the previous unacknowledged yttrium samples from mid-January (YS-1 and YB-1) had relatively low levels of alkali earth substitution for yttrium (10%), were processed at a maximum temperature of 1100 °C, and were both found to be insulating. Now suddenly in Houston, the levels of alkali earth substitution (barium, in this case) rise to unprecedented high levels at the same time the maximum processing temperature drops to 1000 °C, all without explanation (I would direct the reader to Part I of this narrative for a painfully detailed discussion of how these conditions were reached in Huntsville). Despite moving into what is by Chu’s accounts unknown[283] waters, there is a flurry of intense new (actually renewed) activity on yttrium (and beyond to lutetium, scandium, and ytterbium). Strontium is ignored completely, despite the fact that YB-1 was previously no less a failure than YS-1 and should have been considered an even less promising path given its bright green color; YS-1 would have likely been a dark gray.[284] The heavy metal lead is also picked up as a candidate (recall the mercury substitution in Huntsville just days earlier). Oddly (or perhaps not), the only agreement between Meng and Chu seems to be that the coincidences in Houston in the hours after the “good news phone call” were all without knowledge of what had just happened in Huntsville (activities that would, of course, include my three-digit precise compositions nestled in the middle of the relevant documentation).
Seventh, an examination of the order in which raw material calculations appear in Exhibit H is also curious. The second Lu-Ba-Cu-O composition listed in Exhibit G, appearing as LuB-102 on page H55, is formulated before the first, LuB-101, which does not appear until page H59. Similarly, among the ytterbium samples starting on page H57, YbB-102 appears before YbB-101. In both cases, the “102” samples that appear before the “101” samples have 40% substitutions of barium for the rare earth. Thus, even before the first of these samples is tested (independent of whether or not one uses Chu’s compressed all-nighter timeline or Meng’s three-plus day timeline), there is an otherwise unexplained preference for a 1.2-to-0.8 rare earth-to-barium ratio like that of the critical Y1.2Ba0.8CuO4 sample just tested in Huntsville. Of course, the reader might note that the one glaring exception to the curious out-of-order synthesis is with the yttrium-barium combination, where YB-101 (page H55) is actually made before YB-102 (page H56). Again, without the benefit of the Huntsville results, the inconsistency is difficult to explain. However, given that what was effectively YB-102 had already been tested in Huntsville (and now brought to Houston), there was no compelling reason to make it first.
Finally, a comparison of the YB-102 test results with those of the previously examined test in Houston on a Huntsville-made sample may also shed some light on the plausibility of what Meng requires us to believe are two independent YBCO “discoveries” – one by Meng’s account in defense of Chu, the other by Chu in his mid-’90s papers – occurring in the same lab at the same time on materials of the same composition processed at the same maximum processing temperature, and both efforts supposedly under the direction of the same Paul Chu. Turning back to the 30 January “Wu’s #2” test[285] (again recall that all of the tests on Huntsville-made samples were withheld by Houston in the original patent case), note that “Wu’s #2” and YB-102 are tested on consecutive days. Next note that they are charted on what is exactly the same kind of paper (compare the printing in the left margins). Next note the handwriting, especially in the digit “8” in the dates written in the top right corners of “Wu’s #2” and the first of the YB-102 tests. Note “LR-400” that appears at the tops of those same pages, referencing the model of the AC resistance bridge used in the tests. Note the test results, both showing clear transitions starting in the low 90s Kelvin. And, of course, note that “Wu’s #2” is actually tested one day before the 1 February YB-102 test described in Meng’s declaration as the first positive Y1.2Ba0.8CuO4 results. To the list of “sames” we can now add same test equipment, same results, and apparently tested by the same person. I think that I can safely speculate (and with all due respect) that it is a little hard to believe Meng’s suggestion that the only inaccuracy in her original testimony concerned the origins of the yttrium “idea.”
The reader might be asking two questions at this point. First, why would Chu morph a series of successful samples into failures? Second, why would I go to so much trouble to prove that they were actually successful? The first question still mystifies me a bit as well, although there is a statement by Chu in the August 1988 Science article that might offer a clue. Following a description of what appears to be an actual acknowledgment of the failed mid-January yttrium samples (a failure Chu attributes to “inexperienced undergraduate students” instead of to the fact that the compositions and specified processing were unfavorable) trailed by a description of Chu’s plans to assign a research associate to redo the substitutions (plans somewhat fulfilled by Chu’s mythical 29 January all-nighter), the article states:
If the University of Alabama in Huntsville group had not found the yttrium material, Chu says, the Houston group probably would have soon.[286]
Of course, the strongest evidence (were it real) in support of such an assertion would be an ongoing effort with YBCO in Houston prematurely interrupted by the discovery in Huntsville, thus leaving Houston a mere step behind and only as a result of the supposed relative unavailability of yttrium oxide.
The answer to the second question is left as an exercise for the reader.
Returning (finally) to a more strictly focused examination of Chu’s papers, his next dated event is the mailing of the YBCO reports (a second paper examining pressure measurements on YBCO accompanied the first) on February 5th to the Physical Review offices. Note that there is no mention of Meng’s February 1st discovery that prompted our revisit to her earlier testimony or, for that matter, any other event of note on February 1st.
So, we have, by Chu’s account, a 29 January discovery in Huntsville, the “good news phone call” that evening, and a series of confirming tests in Houston, culminating in the submission of a paper that, unless the situation has changed in the last decade or so, is the second most cited paper in the history of Physical Review Letters.[287] While Meng is listed second on that paper, her patent testimony has been shown to describe an independent effort with Y-Ba-Cu-O in Houston that parallels in many ways the events described in Chu’s mid-’90s papers (without actually being those events), all taking place within the same laboratory.[288] She struggles in her deposition to recall the Huntsville team’s involvement as otherwise described by Chu, and her declaration scarcely makes any reference to the Huntsville team. Her testimony climaxes with a 1 February “discovery”[289] in Houston that Chu seems to find completely unworthy of mention. In any case, if one is to believe Meng’s memory lapse, then that would imply that she was not involved in the activities that culminated in the first YBCO paper. I would be curious to know the chances that she would contact the PRL publishers after so many years to ask that they print an erratum removing her name from the list of contributors.
So Where Does Chu Stand Now?
Having examined many of the more prominent accounts, it is instructive to examine Chu’s more recent position on the veracity of these disparate stories. To that end, I have linked here a copy of a 2007 letter written from Chu to a colleague at the University of Houston regarding the 2006 lawsuit with Hor.[290] I will quote heavily, but note that excerpts will be organized topically. In addition to nauseatingly endless yammering on who first belched the word “yttrium,” Chu includes a description on page 7 of his interactions with Robert Hazen in isolating the pure-phase YBCO compound. He includes on page 13 the reference:
The Breakthrough: The Race for the Superconductor, Robert M. Hazen, 1988.
So, Chu would seem to be endorsing Hazen’s book – the story of weeks of work in Huntsville gradually zeroing in on the critical YBCO composition, all micromanaged by Chu and culminating in a 29 January climax (again in Huntsville).
Chu’s letter additionally includes a description of the process by which his aforementioned desk calendar was located:
I am pleased, however, with one helpful event which occurred within the last week – my wife located my calendar from this period which contains my personal entries that expose the falsity of these charges.
and on page 5,
Just a few days ago, my wife discovered my calendar for 1986-87, which I thought was discarded long ago. Ex. 9 includes the calendar pages for December 1986 - March 1987. All entries are written by me during that time period, typically on a daily basis just as shown.
and page 9,
My conception of Y as a substitute for La is clearly set out in my handwriting on my December 1986 calendar on December 18, 19 and 26, 1986. (Ex. 9).
and I will finish with this one on page 6,
Now, my calendar entries confirm the truth, and it should be clear that Meng’s retractions in 2006 were not truthful.
Thus, one should reasonably conclude that Chu believes that, contrary to his account in Hazen’s book that he appears to endorse, Meng’s testimony in the original patent case must have been true – a 1 February discovery in Houston with no role, direct or indirect, by the UAH group and Meng having only the fuzziest of recollections of the 30-31 January testing in Houston on Huntsville-made samples.[291] This is difficult to reconcile with Chu’s description of the excitement of those two days in his mid-’90s papers, among them the excerpt previously examined from History of Original Ideas and Basic Discoveries in Particle Physics. Coincidentally, Chu’s letter just so happens to mention that very paper earlier in the letter (pages 3 and 4):
In 1995, I was asked to write a chapter entitled “High Temperature Superconductivity” for a new book to be entitled “History of Original Ideas and Basic Discoveries in Particle Physics,” which was published in 1996. Chapter 42 is included as Ex. 5. This chapter describes much of my recollection of the events that occurred over the general time period November 1986-March 1987.
Thus, it would seem reasonable to conclude that Chu, before citing Hazen’s book and then expressing chagrin over Meng’s retraction, is endorsing his mid-’90s renditions which have been shown to be fundamentally in conflict with both Hazen’s story and Meng’s patent testimony. Hence, Chu is simultaneously sanctioning Hazen’s story of the micromanaged 29 January discovery in Huntsville, Meng’s 1 February discovery in Houston, and finally Chu’s own first-hand accounts of a discovery now back in Huntsville on 29 January. The latter, devoid of the micromanagement described by Hazen, is festooned with the “excited” and “ecstatic” flurry of activity in Houston on the 30th and 31st, a most memorable sequence by Chu’s words but forgotten by Meng under oath in Chu’s defense.
Sadly, Chu’s letter did not address the NOVA documentary where he played himself in a reenactment of the discovery in Houston, but I think it would be safe to extrapolate at this point his position that all of the mutually-exclusive stories are deemed simultaneously true.
There is one topic that I have been reluctant to include, and I will leave the final decision to my editors. Chu happens to mention in his letter the distribution of the proceeds from DuPont’s purchase of the Houston patent claim in 1988. From page 8 of Chu’s letter can be summarized the following:
- Chu – $239,529.70 (35%)
- Ru-Ling Meng – $137,000 (20%)
- Pei Hor – $137,000 (20%)
- M. K. Wu – $137,000 (20%)
- “Others” – $34,250 (5%)
Yes, that is M. K. Wu listed. Incidentally, his only contribution in defense of the UAH claim was a very short declaration. The reader will have to contact Houston for the details (see Appendix D for an alternative possible source for this information), but I have been told that the $34,250 balance was distributed in portions ranging from about $500 to $7000 to nine other individuals, covering all of the names (save two) listed on the original YBCO paper plus four other individuals. For those who are curious, the missing two were C. J. Torng, the person who physically made the first YBCO superconductor, and myself. I can honestly say that I am proud not to be included in the above listed group.
Very recently (14 March 2015), I came across a Chu preprint on Arxiv.org.[292] Large portions appear to be recycled from past papers, but a few items are of interest, beginning with:
With RBCO [the “123” superconductors including YBCO and variants based upon other rare earths] being the first HTS family to bring down the liquid nitrogen temperature barrier of 77 K and the HTS of choice for applications, as well as the heavy personal involvement by one of us (CWC).
I will simply point out that, from my perspective, it is intriguing that Chu must remind himself and his readers of his “heavy personal involvement.”[293] I would remind the reader that this “heavy personal involvement,” by Chu’s accounts (excepting the Hazen experiment), appears to consist of not a single communication between Houston and Huntsville during the four weeks between the yttrium dispensation and the discovery itself (and even Hazen’s account never mentions any specific information being relayed during that period). Next:
Chu prepared a patent disclosure at the beginning of January and filed a patent application with the US Patent Office on January 12, 1987, which happened to contain the nominal Y1.2Ba0.8CuO4, in which the stable 90 K superconductivity was first observed less than three weeks later by Wu and students in Huntsville. It should be noted that attempts to make nominal Y1.2Ba0.8CuO4 in Houston failed, as shown in the January 13, 1987, entry to our lab book.
I can only assume that the phrase “happened to contain” here is a bit of a Freudian slip (and no, Y1.2Ba0.8CuO4 was not explicitly listed in the application; it was only covered by the vast range of possible compositions). Let it never be said that with each retelling, the YBCO discovery story fails to consistently acquire new and significant elements. In this account of Chu’s over 28 years after the event, the reader learns for the very first time that samples of the critical composition Y1.2Ba0.8CuO4 were supposedly made in Houston weeks before being made in Huntsville.
Of course, the passage seems to serve a very interesting purpose, namely suggesting that from among the huge numbers of combinations covered by the 12 January patent that the critical Y1.2Ba0.8CuO4 composition had been somehow singled out for study. Apparently one is left to infer from this that the work on Y1.2Ba0.8CuO4 was soon thereafter “reassigned” to the Huntsville team. However, the holes in this brand new tale are many.
First, if we combine this mid-January Y1.2Ba0.8CuO4 failure in Houston with the late-January Y1.2Ba0.8CuO4 failure in Houston (from Chu’s mid-’90s papers covered just a few pages back) along with the supposed tasking of Huntsville that also led to a Y1.2Ba0.8CuO4 sample, one can only wonder how many times Y1.2Ba0.8CuO4 might have been tried by the Houston-Huntsville “team” had success continued to be elusive. Of course, Chu’s December calendar did indicate “yttrium has to work,” which would perhaps explain the seemingly endless attempts with Y1.2Ba0.8CuO4 even as it, by these many very confused accounts, supposedly failed again and again.
Of course, the reader should further recall from Chu’s mid-’90s papers that, by his account, yttrium oxide was “newly-arrived” in Houston as of the 29 January “good news phone call” and that, again by those earlier accounts, the decision to make samples with it came after the phone call.[294] Of course, it is the mid-’90s papers that were in error, Houston did in fact receive yttrium oxide on 14 January, and yttrium samples were immediately fabricated. But which yttrium samples?
In all of the documentation submitted by Houston in support of their claim on the Y1.2Ba0.8CuO4 material there is no such formula appearing on any page marked January 13, 1987.[295] Given the date Chu assigns, his reference is almost certainly to the YB-1 composition shown on page H47 from Meng’s notebooks (clearly marked 13 January 1987, in fact),[296] the test results (insulating) of which appear on page Meng’s page H476.[297] However, the composition listed there (sample YB-1) is Y1.8Ba0.2CuO4, one fourth the barium of Y1.2Ba0.8CuO4 and essentially a straight substitution of Y for La in the 30 K La1.8Ba0.2CuO4 superconductor to yield a composition that, at equilibrium, would be completely devoid of the YBa2Cu3O7 90 K superconducting phase. In other words, the “failure” described by Chu was because the composition of the sample that was actually made was wrong and samples with a sufficient composition were never made, not because of any ineptitude on the part of certain undergraduate students.[298] Chu continues,
On January 12, 1987, we observed a large diamagnetic shift or Meissner signal up to ~96 K in one of our mixed-phase samples, as displayed in Fig. 7, representing the first definitive superconducting sign detected above the liquid nitrogen temperature of 77 K. Unfortunately, the sample degraded and the diamagnetic signal disappeared the following day. No effort of ours in the ensuing two weeks succeeded to reproduce and stabilize this high temperature superconducting signal. Chu decided to write up and report details of the experiment and let other better equipped groups stabilize and identify the high temperature superconducting phase.”
The many details make it clear that this is in reference to sample J-31. Despite the fact that the results (as shown earlier) were clearly driven by the background test (as confirmed by Hor), Chu continues to boldly declare the results “the first definitive superconducting sign detected above the liquid nitrogen temperature of 77 K.”
The above also represents one of many direct and indirect suggestions that the YBCO discovery came as a result of a concerted two-month effort to “stabilize the high Tc phase” in La-Ba-Cu-O by replacing lanthanum with yttrium. Other examples include Chu’s “Superconductivity above 90 K” paper covered earlier[299] along with the various notes on “stability issues” in the three presentations from Denver,[300] Urbana,[301] and St. Louis.[302] The best example would undoubtedly be the mid-1987 paper where Chu revealed evidence for the never again seen 240 K superconducting phase in YBCO.[303] In that paper we noted his statement, “It took about 2 months (November 25, 1986, to January 29, 1987) to stabilize the 90 K superconducting phase.” November 25th is in reference to sample #1b (to be covered in Appendix A) whereas January 29th is obviously a reference to the YBCO discovery in Huntsville. Of course, this raises the question of why, if the supposedly unstable superconductivity that was seen in Houston in samples like #1b and J-31 only occurred when barium was included in the composition and was never seen when barium was absent, would the Huntsville team, purportedly part of this effort to “stabilize the 90 K superconducting phase” first supposedly seen in sample #1b, test, make a mere two samples with barium (3 and 19 January) leading up to the 29 January discovery.
I will point out here that I do believe that Chu was indeed probably motivated by the sample #1b (see Appendix A) and J-31 observations (the “overzealousness” described by Cava;[304] “excitement” as it is repeatedly described by Chu), and I now wonder if Wu was, in fact, encouraged by Chu (or tasked, depending upon one’s perspective) to continue emphasizing the strontium materials, thus keeping us at a safe distance from what was believed to be possible with the barium compositions.[305]
And Wu?
Concerning Wu’s more recent position, scarcely a month before the above letter was penned by Chu, he was interviewed by Taiwan Today on the 20th anniversary of the YBCO discovery. In the article,[306] he is quoted as saying (my emphasis):
Around mid-January, we came up with an idea that we could try a new combination of materials to form a new compound. Ashburn did some calculations, and around January 27, we made a sample. The first sample we made became superconductive. The whole discovery process seemed to be very simple. It just came in one shot. At that time, we made two samples, two different compositions, and one of them just happened to work.
Except for generous employment of the pronoun “we,” the article was remarkably accurate on several points. Conception occurred in mid-January. I “did some calculations.” A sample was made “around January 27” (the 28th actually). The first sample was superconducting (so much for Hazen’s story). And my favorite has to be, “the whole discovery process seemed to be very simple.” No doubt true, from Wu’s perspective. Finally, “we made two samples, two different compositions, and one of them just happened to work.” Barely two months after the discovery, Wu had to be reminded of his failed Y1.8Sr0.2CuO4 sample (see Part I), but twenty years later his memory seemed to be working just fine. It is unfortunate that it took so many years for Wu to drop most of the qualifiers from his descriptions of my contributions. In 1988, Wu had characterized my role this way (my emphases):
Jim has done a lot of work.[307] He did sort of summarize some of the data we had and found some correlation between combinations of different ions and transition temperatures.[308]
To my knowledge, Wu’s occasional mention of my “calculations” and “correlations” are the only specifics he ever gives of the process by which the YBCO superconductor was discovered. Oddly, this statement, some 20 years after the events, appears to be the first time Wu credits me with some cognitive act not explicitly described as being prompted by supposed discussions between us. Having shown in Part I a relatively cohesive path by which the critical composition was formulated, complete with the sources (not discussions) that illuminated that path, I cannot specifically recall any information communicated to me by Wu that would have been essential.
I would also add that, outside of the now-abandoned 1 February discovery story delivered by Houston in the patent interference, the above-referenced Taiwan Today and Huntsville Times articles appear to represent the only instances where one of the UAH/UH "team" members publicly recognized specific technical contributions to the discovery by another team member.
Summary
With that I will try to summarize some of the key points of my narrative:
First, arguments over who first mouthed yttrium are, quite simply, nonsense. I have even included a much earlier paper (Er-Rakho et al., 1981[309]) on one of the very same compositions under investigation that contains references to yttrium substitutions for lanthanum. Recall my Four Corners Monument analogy – in my mind, yttrium has replaced tellurium as forever synonymous with the beautiful state of Colorado. The evidence indicates that everyone working in the field at the time at least considered yttrium before either trying or rejecting it, and all those who tried it, save one, failed. Recall the first two yttrium samples made in Houston (mid-January[310]),
- Y1.8Sr0.2CuO4
- Y1.8Ba0.2CuO4
processed at 1100 °C, both of which were indicated as insulating, and after which yttrium appeared to have been abandoned by Houston. Compare these to the first two yttrium samples made in Huntsville on 28 January:
- Y1.8Sr0.2CuO4
- Y1.2Ba0.8CuO4
processed at 999 °C and the last one of which proved to be a 90 K superconductor. Clearly, the real keys were 1) pairing yttrium with barium (not strontium), 2) a relatively high proportion of barium, and 3) a sufficiently low processing temperature. Part I of this manuscript describes precisely how all of these conditions converged in Huntsville.
Second, Wu’s confession, “Yb or Not Yb,” the systematic errors in the X-ray diffraction data, cherry-picking of Houston data submitted in the patent interference (in particular, withholding results from tests in Houston on samples originating in Huntsville), Meng’s data redaction/unredaction on the same page with the suspicious (Y0.6Ba0.4)2CuO4 entry, her “perjury affidavit” and J-2/J-31/J-36 conglomerate, Chu’s blanket endorsement of hopelessly conflicting accounts, and, of course, sample J-31 would suggest that I had come into an environment where transparency is to be avoided, consistency is not a virtue, and “truth” (or something vaguely akin to it) is 90% perception, 10% reality.
Third, in contrast to the precise details I have offered, Chu provides “high pressure” pointing the way to the rest of the periodic table, a series of “shotgun” patent applications, Sample #1b (see Appendix A), and Sample J-31 (the test whose background measurement can turn almost any material into a 100 K superconductor). Regarding the two samples, Chu never explicitly states what was inferred from those tests that influenced activities in Huntsville, and he never describes any communications between Houston and Huntsville subsequent to the supposed yttrium fart around the New Year. While suspicious formulas appear in the Houston notes, Chu and his colleagues seem to be content to allow others to only infer that specific compositions and processing were somehow communicated to Huntsville (see Appendix D for Chu's commentary on this matter). If indeed they were, then why is the topic of the first mention of yttrium so belabored if a specific composition and processing would so easily trump it (see Appendix D for a further beating of this dead horse)? In any case, the Huntsville team spends January testing samples whose compositions or results are never mentioned by Chu in his accounts. Then suddenly the discovery happens, at a time (January 29th? February 1st?) and place (Huntsville? Houston?) that seems strangely dynamic. At best, the stories originating in Houston are imprecise. Their worst the reader is free to assess.
Finally, I have presented, in painful detail, the actual process by which Y1.2Ba0.8CuO4 was conceived, a simple and unambiguous path from La1.8Sr0.2CuO4 to the pivotal Y1.2Ba0.8CuO4 composition (the right elements in the right ratios), bypassing the insulating Y1.8Sr0.2CuO4 and Y1.8Ba0.2CuO4 compositions. Wu gives his support of this path as the rationale behind the discovery by providing an excellent nutshell description in his doctored lab notebook page.[311] While not great science by any measure and perhaps marginally science at all (see my dissertation chapter for my self-critique), it is however the truth. Its application during that period is supported by the patterns in the Huntsville lab notebook and are further strictly established by the curious compositions appearing in Meng’s notebook. A specific combination of elements in specific ratios combined with a serendipitously cool furnace yielded the 90 K discovery. My story is the same today as it was 28 years ago, detailed, self-consistent, precise (even to 3 significant digits), and corroborated by the Houston lab notes.
Having established both the existence and relevance of my ideas at the time of the discovery, I will briefly interject here a quick analysis of the degree to which my ideas might be of the kind that a competent scientist would consider. I would strongly urge the reader to revisit from Part I the excerpts from my dissertation concerning the arguments and presumed constraints by which the original YBCO composition was derived from (La0.9Sr0.1)2CuO4‑y, then consider the following from page 202 of Hazen’s Breakthrough (my emphases):
In many structures it is possible to replace pairs of atoms simultaneously, providing [sic.] the atom sizes are similar and the sum of the charges is the same. In the 1-2-3 [90 K superconducting YBCO] phase, for example, the element pair barium plus yttrium (denoted Ba2+ plus Y3+) might be replaced in part by the atom pair potassium plus zirconium (K+ plus Zr4+). I [Hazen] alerted Chu to this possibility as well as a number of other coupled substitutions.
Concerning Hazen’s qualifications as a competent scientist, he is a graduate of MIT and Harvard and was, at the time of the above writing, a research scientist at the Carnegie Institution of Washington’s Geophysical Laboratory. In comparing the above passage to the ideas as outlined in my dissertation and anchored to the time of the discovery by Meng’s notebooks, Hazen’s thinking is found to be an almost precise replica of the steps by which I made a pairwise replacement (Hazen’s “coupled substitution”) to derive Y1.2Ba0.8CuO4 from La1.8Sr0.2CuO4. The differences between Hazen’s logic and mine are inconsequential. In Hazen’s passage, maintaining the charge is given priority over preserving the size. In my case, because I weighed the facts that La1.8Sr0.2CuO4 contained mixed-valence copper, was known to support a wide range of La3+-Sr2+ ratios, and could sustain large numbers of oxygen vacancies, I instead gave priority to preserving the composite size of the atoms (specifically volume) over maintaining the charge.
It is furthermore almost eerie that Hazen lists the pair K+ plus Zr4+ when my dissertation specifically lists “the monovalent alkali metal ions Na+ and K+” and “the largest quadravalent cations (e.g., Ti4+, Zr4+, and Pb4+)” as I considered but rejected substituting lanthanum and strontium with a pair of ions with the higher valence difference. My flirtation with potassium and zirconium can also be seen in my scratch notes from January 1987.[312]
In any case, Hazen echoes almost perfectly (albeit unknowingly) the process by which superconductivity in YBCO was achieved, a very simple idea nestled in what should have remained a very simple story. No weasel-worded descriptions of how high pressure measurements pointed the way to ever smaller ions while somehow trumping what actual substitutions were demonstrating (recall “Ba-rich LaBCO” aka J-31). No endless trial and error, gradually zeroing in on the target composition. No fleeting, tantalizing hints of superconductivity from unstable materials. No “J-31/J-2/J-36/Background/Empty probe” mass of confusion. No drama, and no crack team of lab rats toiling at the behest of their brilliant leader. Just an unfortunate 22-year-old first-year graduate student[313] who still today struggles to separate his love for science from his disdain for scientists.
Request for Reconsideration
It would be difficult to close this manuscript without a brief analysis of the IEEE Milestone that prompted its writing. From “Milestone Proposal: High-Temperature Superconductivity,”[314] a web page dedicated to the justifications for the award, are found the following statements:
On this site [Science and Research Building 1, University of Houston] in 1987, yttrium-barium-copper-oxide, YBa2Cu3O7, the first material to exhibit superconductivity at temperatures above the boiling point of liquid nitrogen (77k), was discovered.
The YBCO compound was first synthesized and tested for superconductivity in this building.
January 2012 marked the 25th anniversary of the discovery of superconductivity above the liquid-nitrogen temperature by Paul C.W. Chu and coworkers at the University of Houston.
The first reference cited for the final statement above is the original YBCO paper “Superconductivity at 93 K in a New Mixed Phase Y-Ba-Cu-O Compound System at Ambient Pressure” published jointly by the Huntsville and Houston groups.
Taken as a whole, the above language presents great ambiguity concerning whether the citation is for the discovery in the original mixed phase Y1.2Ba0.8CuO4 material (as indicated by the above implied January 1987 discovery date in combination with citation of the original YBCO paper) or the pure YBa2Cu3O7 compound (as explicitly listed in the first statement).
Since the synthesis of Y1.2Ba0.8CuO4 yielded a mixture of the pure-phase YBa2Cu3O7 superconductor and the insulating “green phase” Y2BaCuO5, the only self-consistent interpretation of the collective statements is to interpret the reference to YBa2Cu3O7 as applicable to any mixed phase materials that include YBa2Cu3O7 as a component. If that is the correct reading, then the Milestone wording stands in conflict with Chu’s own accounts that clearly state that Y1.2Ba0.8CuO4 material was first synthesized and tested in Huntsville (with the curious exception of the recent 2015 Arxiv.org preprint discussed previously).
A second interpretation is that all of the references in the Milestone strictly concern the pure-phase YBa2Cu3O7 superconductor, an interpretation inconsistent with the January date and the reference to the original YBCO paper. Chu identifies Hazen’s Geophysical Lab team as identifying the stoichiometry and structure of the YBa2Cu3O7 phase.[315] By Hazen’s reckoning, the compounds within the mixed phase material were identified in eight days (20-27 February 1987, see page 145) from the time the first mixed-phase samples were received.[316] It should be noted that much of that time was rather wasted on the Y2BaCuO5 green phase,[317] prompted by Chu’s urging that the superconductivity might be an “interface phenomenon… at the grain boundaries between [the] two phases” (page 127), a highly unlikely (if not ridiculous) notion given that the superconductivity in the earlier 2-1-4 materials was already known to be a bulk property of a single phase.[318]
According to Hazen, the information was relayed to Chu on February 27th (Hazen, page 147). Chu’s Novel Superconductivity paper (page 584) corroborates the date and references the Phys. Rev. B paper principally authored by Hazen.[319] Thus, the date of the first pure-phase YBa2Cu3O7 test clearly cannot be reconciled with the January reference in the Milestone citation. Ironically, if one recalls Meng’s testimony in the UH/UAH interference claiming a 1 February discovery, even a January date for the original mixed-phase Y1.2Ba0.8CuO4 material would be at risk for Houston.
In any case, with regard to identification of the pure phase YBa2Cu3O7 superconductor, Chu concedes in his IEEE Transactions paper,[320]
Several other labs obtained similar results independently at about the same time.
Incidentally, several of the “other labs” mentioned by Chu above became part of a four-way patent interference over who first identified[321] the pure-phase YBa2Cu3O7 compound.[322] For the record, Houston did not win.
The only logical conclusion here is that with the formula of what Hazen calls “Chu’s secret superconductor” in hand (pages 93 and 125), specifically Y1.2Ba0.8CuO4, identification of the pure-phase material and confirmation of its properties was an inevitability timed almost as well with a stopwatch as with a calendar. If the confirmation were indeed an achievement worthy of IEEE’s Milestone recognition, then one can only wonder why Chu would make no mention of such tests in his various stories and proceed directly into the events by which most of the rare earths were determined to be near equivalent substitutes for yttrium.[323]
Thus, one critical question lingers: Is there a single credible event that can be shown to have transpired at the University of Houston that justifies the IEEE Milestone? To my knowledge, the options are these:
1) J-31: Dubious indications of superconductivity at ~100 K. Sample contains no yttrium.
2) “Initial mention of yttrium and/or yttrium substitution for lanthanum:” First, imagine in the excitement of that period leading up to the discovery just how many times and by how many people yttrium would have been mentioned. The debate itself (see Appendix D for more) is comical. Second, I would have to attribute the original idea to Er-Rakho et al. Recall the early La3Ba3Cu6O14 composition made in Houston, sent to Huntsville, and traceable to the 1981 paper by the French chemists that mentions an yttrium-for-lanthanum substitution.
3) The 12 January Houston patent application: Given early YBCO failures in Houston, how long would one suppose it would have been before Houston would have doubled back in the course of examining 26,775 combinations of elements to try their YBCO samples with different ratios? On the subject of YBCO samples in Houston…
4) Samples combining yttrium, barium, copper, and oxygen tested in Houston before the first such test in Huntsville – tests that all testimony suggests failed and until the very recent Arxiv/Physica C paper were of formulas and processing unfavorable to producing any of the superconducting phase.
Concerning the last option, it only recently struck me how carefully worded is the second of the three statements introduced at the start of this section – “The YBCO compound was first synthesized and tested for superconductivity in this building.” If one liberally interprets “the YBCO compound” to mean any compound of yttrium, barium, copper, and oxygen (such as YB-1) and if one interprets “tested for superconductivity” as not necessarily meaning successfully tested, then the statement is true to the extent that it can be shown that Bellcore and the University of Tokyo did not, in fact, also unsuccessfully test yttrium barium copper oxide samples prior to the 13th of January (incidentally, Bellcore claimed 3 January[324]).
I must close this chapter by recognizing that the IEEE History Committee has already made a substantial effort towards investigating many of the issues I have raised. As described to me, IEEE's History Committee Chairman, Mr. David Burger, met with Chu and Houston legal counsel on 17 January 2015 in an attempt to reconcile some of the more prominent discrepancies. Unfortunately, the reception was "openly hostile," and there was no discussion of the events surrounding the discovery. In defense of the Houston position, they did note that the ongoing Hor-Chu case was a limiting factor. Mr. Burger more recently granted me permission to quote his recent message summarizing the current IEEE position:
March 16, 2016
Dear James,
As a sequel to the IEEE Milestone Challenge received, the Committee did review the veracity of the 2014 Houston Milestone in early 2015, and actually met with Chu in Houston to corroborate key facts. The outcome of the IEEE meeting degenerated with Chu’s legal counsel interrupting, leaving the meeting outcome inconclusive. The IEEE History Committee does not have the resources to conduct an investigative probe as requested, and it was seen the original submission by the IEEE Houston Section still supported the UH Milestone. The IEEE Committee position was then to leave the Houston Milestone unchanged. Cognisant of Dr. Ashburn’s request for a reconsideration, the IEEE History Committee has since adjusted the internal processes used to assess future Milestone recognition. While the IEEE History Committee is aware that Dr. Ashburn is uncomfortable with the outcome, the History Committee has in good faith attempted to manage the complexities surrounding this historic achievement.
Yours Faithfully,
David Burger
IEEE History Committee, Milestone Coordination Chair 2016
In the interest of providing the means by which all the concerned voices could be heard, IEEE has graciously accommodated a "First-Hand Article" on their Engineering and Technology History Wiki (ETHW) site -- this article. As ETHW is the near-perfect forum by which a story with so many accompanying documents can be told, I cannot possibly express the full extent of my appreciation.
Closing
My apologies to the reader for the length of this saga. It is indeed a Byzantine tale, but I can assume no responsibility for its limitless complexity. Let it be understood that I feel no compelling need to convince anyone of anything. To the reader who does not believe my story, so be it. It stands as my record of the events.
I also recognize that this narrative may not be well received by all, particularly its principal characters. Having already addressed Ruling herein, I should address a few others.
It was a relief to establish a connection with Pei Hor. He has proven himself to be a sincere and honorable man and has kindly and patiently responded to occasional questions over the years as I sought to reconstruct some finer details of the timeline in Houston.
Wu provided me the opportunity to do some fascinating work in Huntsville. He is a capable teacher, as well. He offered little resistance to my automation of our laboratory and, in addition to Y1.2Ba0.8CuO4, tolerated many of my other harebrained ideas. With the YBCO discovery, Wu found himself, shall we say, between “a rock and a hard place.” He was a young professor bombarded by people telling him what he needed to do. I cannot say that I, in his position, could have handled it much better.
To Chu, I struggle with what to say. I penned the aforementioned letter on August 16th of 2013 seeking to “open up a dialog” to better “understand and reconcile many things.” Over the course of several subsequent emails, phone calls, and voicemails, I failed to establish a meaningful connection. In his defense, in a pair of two-line emails he did send an alternative phone number and recommended times to call, but several attempts reached only his answering machine. I then emailed his assistant Troy Christensen on 11 October 2014, noting my failures to make contact at the recommended times and asking for alternative windows. He replied two days later that Chu was on travel and that I would receive a subsequent email concerning best times to call. The email never came. I eventually emailed all of the phone numbers by which I could be reached but received no response. After repeated attempts via multiple channels spanning over a year, I conceded failure.
Oddly, back in 2001, Chu tracked me down at my then place of employment (15 years after the discovery) and left a most unusual message on my voicemail (audio track linked here[325]). Recall that I had only met him about four times in my life. He knew little to nothing about me, and I knew similarly little about him. Nevertheless, I received a voicemail thanking me for all my “work[326] previously on high temperature superconductivity.” I can only speculate as to what motivated him to do so.
To the more curious reader, I would highly recommend contacting the members of the original groups, both in Houston and in Huntsville. If assistance is needed locating them, I will gladly help. I am not suggesting that one will now necessarily receive consistent and mutually corroborating answers, but with the information contained herein (including the many linked documents), I think that sufficiently probing questions should be easy to formulate.
I have been asked in the past how this entire episode could have “gone so wrong.” Even though it is, for me, a very emotional issue, I have tried in this manuscript (sometimes inconsistently, I must confess) to stay close to the facts. In answer to the inquiries, I would defer again to Robert Cava. In clarifying the quote to follow, I should first note that in addition to Chu’s claims from June of 1987 of possible superconductivity at 240 K,[327] Wayne State briefly made headlines in April of that year claiming signs of superconductivity in YBCO at the same temperature.[328] I would further temper Dr. Cava’s very candid assertions by noting that it is not entirely inconceivable that all parties involved with the current YBCO saga have been sincere by some measure. Thus, I would emphasize from the excerpt below references to “mistakes in judgment” and “overzealousness” and note that it is often the data that “lies.” With an additional warning that Dr. Cava’s sarcasm is sometimes subtle, the following is taken from his paper entitled “Oxide Superconductors” (my emphases):[329]
The sidetracks, sideshows, false alarms, and downright fraudulent claims in the history of the field of high-Tc superconductivity have been an ever-present and often important part of the story since the field’s inception. Some of the claims of the observations of very small amounts of very-high-Tc materials (e.g., at the recurring apparent Tc value of 240 K) remain real mysteries to this day, because no obvious flaw in logic or mistake in the experimental procedure is evident. These observations have never been reproducible in other laboratories following the reported procedure – this is logical, in a sense, because the extreme superconductivity is always present at the 1 part in 10,000 level or lower. It is impossible to estimate, however, how many hours have been spent by the materials community attempting to duplicate what appeared to be spectacular results and which were mistakes in judgment, inadvertent error, overzealousness, or outright lies... In the first years of research in the field, there was virtually no negative feedback by the scientific community for researchers who made these wild claims. The attention such claims got in the popular press created considerable havoc and never did the retraction of the story eventually shown to be incorrect ever get the type of attention the original announcement received...
Koichi Kitazawa coined the perfect term for these announcements of outrageously high transition temperatures in 1987: USOs. This is actually a triple entendre: USO stands for “unidentified superconducting object.” The obvious analogy to UFO conjures up the appropriate implications about their verisimilitude, and the character of the people who chase them, and, finally, USO means “lie” in Japanese…
Some of the origins of USOs and confusing claims are presented in the following… Experimental error or lack of scientific judgment is always [the source of] the most spectacular claim; there are many examples of them in the literature... Many are determined to be obvious nonsense after looking at the data for one minute, but, for some, it is more difficult to discover the mistake.
Cava then proceeds to describe issues with thermometry (see Appendix A) and other missteps that yield flawed tests and inflated conclusions.
Obviously, I anticipate a very unfavorable response should this document ever be published. I have made every possible attempt to carefully include all of the evidence underlying my assertions (very often by citing Houston documents). The relatively few places where I have speculated have been carefully noted, and I have sought to limit my speculation to only what is highly plausible.[330] Over the years, Chu has had several platforms upon which to tell his version(s) of the story, and he has even written me into several of them. In so doing, he has invited my rebuttal. He has spoken and written of events in Huntsville (in forms either devoid of detail or simply fictitious) and has so justified my commentary on events in Houston. He has conceded that I was at least physically close to the activities culminating in the discovery; surely that makes my perspective worthy to be heard.
While the “consensus account” from Houston (i.e., the assemblage of details for which there seems to be general agreement) is relatively accurate, it is also woefully incomplete, and the omissions naturally lead the general reader to inaccurate conclusions. The very same omissions, largely as gaps in their knowledge of the activities in Huntsville, seem to have additionally led some of the Houston team members to similarly inaccurate deductions. In support of those erroneous conclusions, great significance has been assigned to a scattered assortment of events in Houston that, in fact, had precisely zero causal relationship with the discovery. At the same time, the events in Huntsville in the gap between the supposed New Year’s yttrium utterance and the 29 January “good news phone call” are largely ignored (in marginal defense of Chu and his colleagues, they never asked). Chu and those speaking on his behalf fill the void with his 12 January shotgun patent application, uncertain dates for ordering and receiving yttrium oxide, sample J-31, and a one-of-its-kind story communicated via Robert Hazen (Chu’s one failed attempt to fill the Huntsville gap) of the Alabama team testing samples “relocated” from Texas and slowly zeroing in on the optimal YBCO composition.
If there is anything that can be shown to be inaccurate in what I have written, I will make the appropriate corrections. If there is conflicting evidence that needs to be recognized, I will not hesitate to add it and acknowledge any apparent inconsistencies. I would only note that alternative stories that attempt to describe how Y1.2Ba0.8CuO4 was formulated need to explain away Wu’s doctored lab notebook page (and accompanying confession) and, in particular, the curious 3-digit-precise compositions in the Meng notebooks. I would only ask that anyone inclined to respond to my account in a negative way first have an appropriately qualified technical person review what I have written (a solid understanding of freshman chemistry and physics would be among the minimal requirements).
I will finally and perhaps mercifully close with the assertion that I am no “Superman of Superconductivity.”[331] [332] I make no claims to having formulated millions, thousands, or even tens of superconductors. I claim responsibility for only one.
And I have never been anyone’s “pair of hands.”
Postscript
After what is otherwise a rather ugly story, a bit of levity may be an appropriate way to finish. I recently tracked down Steve Kelber, the attorney who handled the case for UAH for all but its earliest first leg. After answering a couple of questions for me, he volunteered this story of how the UAH patent application just barely came to be.
By Kelber’s recollection, someone at the university (I am guessing Charles Lundquist from the Research Institute) had called the law firm (Oblon et al.) “late on a Thursday [and had] come down immediately to talk about an exciting new invention.” Kelber was in Japan at the time, and attorney Vince Sunderdick was sent to meet Wu. I have been told (via another source that escapes my recollection) sketchy details of a confrontation in a Washington, DC hotel room, but I have only an uncertain recollection of a detour by Wu upon our late-January departure from Houston.
Kelber describes Wu’s reluctance to share the chemical formula of the superconductor.[333] By some means, Sunderdick already had most of the details of the formula, including the final three elements. As Kelber recounts, Sunderdick says to Wu, “Okay, it’s a barium[334] copper oxide, right? So let’s write Ba-Cu-O. What’s the remaining element?” Wu refuses to answer. Sunderdick had already an “x” to represent the barium ratio (recall that the high barium concentration was critical to the discovery). Kelber describes what happened next this way:
So Vince says, “All right, let’s just call it Y,” meaning he would leave it a variable. Wu’s mouth drops open: “How did you know?” Vince: “How did I know what?” What should have been a major advance kind of got lost in nonsense, I am afraid.
I am inclined to think that stories like this are simply too bizarre to have simply been made up.
One final tidbit: a calculation of the atomic weight of YBa2Cu3O7 is an interesting exercise, and let it never be said that I lost my sense of humor through all of this.
Jim Ashburn
December 2015
Appendix A, Sample #1b
In addition to Chu’s J-31, there is a second, albeit less notable Houston sample that has similarly been the subject of many retellings. Like J-31, sample #1b also appears to have made its print debut in Chu’s June 1987 Novel Superconductivity paper in the form of Figure 1, a chart of a resistivity test showing a transition starting at 75 K and ending around 45 K.[335] Following J-31, #1b appears in “Superconductivity Above 90 K and Beyond” along with a background image of the raw data.[336] A larger and clearer version is found on the page marked 804 in “High Temperature Superconductivity.”[337] The chart sans raw data is shown in “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc”[338] as well as the three presentations shown earlier.[339] [340] [341]
Both Hor and Meng make reference to sample #1b in their 2006 affidavits, not necessarily by name but the descriptions can be easily matched up with the details provided. Meng states (grammar errors are original):
On November 25th we observed a transition temperature around 70 K in a LaBaCuO sample made by Pei Hor. Unfortunately, the sample was not stable and we would not repeat the results. The results indicated that there were higher temperature superconductors existed.[342]
Meng’s notebooks record this entry (page stamped H486):
- 26 NOV 1987
- 1B Tc 75 – 50 °K [sic.] ?!
Oddly, while there are no small number of cases in Meng’s notes where 1987 was inadvertently recorded as 1986 during the first several weeks of the new year,[343] this entry marks a rather bizarre case where Meng seemed to have been anxiously anticipating 1987 five weeks before its arrival.[344]
Moving along, Hor provides an even more detailed description:
For instance, on Nov. 15, 1986 [date of processing, not testing] I sintered a Ba-La-Cu-O sample on a copper substrate and observed the indication of Tc ~ 70 K, the very first high Tc produced and observed in our laboratory. I measured the sample under magnetic field and followed the transition 4 times. It was a genuine superconducting transition but Tc was unstable and degraded at the 4th measurement.[345]
Before proceeding, I will state here that the observations reported by Houston may have been real. However, I cannot help but be reminded of Chu’s chart from July of 1987 (covered earlier) showing the resistivity test on an yttrium barium copper oxide sample with supposed superconducting transitions at 175 K, 220 K, and 240 K.[346] [347] [348] Again, not one of the purportedly unstable phases was ever duplicated despite the fact that YBCO is among the most studied materials in history. In my opinion, unstable electrical contacts or bad thermometry should always be the preferred first interpretation of what appears to be unstable superconductivity at world record temperatures.
In comparing the charted results to a reconstruction of the raw data, I will draw upon the larger, clearer copy from the page marked H1116 of Meng’s records,[349] reproduced here:
Resistivity test results on sample #1b as taken from Meng’s copies.
As Hor describes, sample #1b was examined at least five times, and the original chart verifies this, showing fives sets of curve segments with corresponding scales and offsets. The first set is simply numbered. The second set is indicated by number and a single “prime.” The third set has two primes, and so on. The original chart would have been in color with various plotter pens as indicated (green, red, black, blue, and black again). Despite the absence of color in the photocopy, with some care it is possible to reconstruct from the tangled mass the relevant parts of the data, especially if one carefully looks for continuity in the lines between segments and notes the relative weights of the various lines (some are darker and thicker, others thin or spotty).
As described by Hor, the tests are of resistivity under a magnetic field, a common test for confirming superconductivity. The bottom of the Denver chart notes “H=0” and “H=350 mV” (presumably the voltage applied to the magnet), indicating that the two sets shown are the first (no indication of the voltage applied to the magnet on the original data chart) and second (explicitly labeled “H=350 mV” on the original data chart).
The first curve from Chu’s chart is the one marked with numbers only (no “prime”), indicated as “green,” and presumed to be under no magnetic field (H not indicated). The second curve charted by Chu is the one in the original data with the marked temperatures (single “prime,” red, H=350 mV), except for the segment from 45 K to 55 K which is marked on the first curve instead. As a result, the person reading the chart appears to have swapped the segments in that temperature range. My reconstruction below will show the correct reading. Temperatures can be found for the first curve by simply noting the x-coordinate of the corresponding points on the second curve.
Applying the process described previously in the section on Sample J-31 and converting voltages to resistance by dividing by the current indicated at the top of the chart (“I=1 mA”) yields the following graph for the first two sets, overlaid on the rendition of the sample #1b chart taken from page 11 of the Denver presentation:[350]
Reconstruction of 25 November 1986 Houston resistivity test on Sample #1b.[351]
The overlay is nearly perfect, except that on the green curve, I had to truncate my reading of -3.9 inches to precisely -3 inches to achieve the match at 60 K.
The alert reader may have already observed that while Chu’s charts span a vertical scale from 0 mΩ to 1.6 mΩ, the reconstruction runs from -1.1 mΩ to +0.5 mΩ. From the otherwise matching tick marks, it is clear that the vertical bias difference is precisely 1.1 mΩ, the y-axis minimum that would be automatically assigned by most charting software. Since resistance is obviously never a negative quantity (a result that, if real, would have been even more remarkable than superconductivity itself), the published charts appear to have been arbitrarily offset by this 1.1 mΩ to yield a more credible result.[352] Chu was apparently so confident of his “nearest convenient round number” bias adjustment that he describes the following:
An even more exciting observation was made only four days later on November 25 in another multiphase sample of LBCO, i.e., a large R-drop by a factor of 80 at ~ 75 K.[353]
The “R-drop factor” here is the ratio of the resistance at the start of the transition (~75 K) to the resistance at the end of the transition (~45 K). By applying a temperature-independent 1.1 mΩ bias to the sample #1b data as I read it yields R-drop factors of 143 (-99.3%) and 46 (-97.8%) for the first two passes. Given that this quantity would logically be a positive number, a geometric mean would seem to be warranted, yielding an average of 81 (-98.8%), consistent with Chu’s otherwise dubious claim. The variation in my estimates corresponds to +/-0.1 inches (one tick) on the original chart.
While, to my knowledge, there is no simple means to compensate for biases in a DC resistance measurement (the Denver chart indicates the test as “DC R-T”), the vertical bias does not fully discredit the data. Chu’s primary purpose seems to be to simply find evidence for superconductivity, and, as long as any background signals are relatively smooth, this can be achieved. Thus, the bigger question here becomes the credibility of the temperature measurement.
The reader may have noted in the analysis of J-31 that the horizontal scale and offsets in that raw data was recorded in millivolts, specifically thermocouple voltage levels. In the Huntsville lab, thermocouples were used for temperatures down to about 20 to 30 K. Below that range, thermocouple voltages tend to flatten, resulting in much larger temperature uncertainty. Consequently, a resistance thermometer was used in the UAH lab for optimal measurements below 20-25 K. The reader will note here the horizontal offsets and scales on the original sample #1b data recorded in Ohms and of a range and behavior consistent with such thermometers.[354] While there are several resistance thermometers available (especially today) with decent performance even at much higher temperatures, the very flat behavior of the thermometer employed in the sample #1b test indicates that it is likely not among those designed for the warmer ranges.
In all fairness, the apparently poor selection was likely no error in judgment. Prior to 1986 the Houston lab had little cause to ever anticipate testing a superconductor beyond 30 K. Moving into the very early stages of testing these new materials, they were less than ideally prepared for thermometry at the higher temperatures.[355] The case here for sample #1b is that the Houston apparatus for measuring resistivity under a magnetic field (a test relatively unique to superconductors[356]) appears to have been configured only for sub-30 K measurements. Fortunately, Chu describes how this problem was addressed.
Upon having completed my earliest draft of this appendix, I came across one of Chu’s more detailed accounts of the sample #1b story (“High Temperature Superconductivity”), precipitating the addition of several excerpts from it to this appendix. Leading directly into the above passage on the “R-drop factor,” Chu writes (my emphasis):
…on the evening of November 21, 1986. It is hard to believe that the greatest experimental challenge at this time was the thermometers, which were all valid only below 25 K. We had to calibrate one of the Ge-thermometers against a copper-constant [sic.] thermocouple and a chromel-alumel thermocouple to be sure that the temperature was correct. An even more exciting observation was made only four days later on November 25…[357]
Chu’s assessment is consistent with my own, that these thermometers are “valid only below 25 K.” Where Chu and I would differ is on the idea that a device accurately characterized as “valid only under 25 K” can be so easily calibrated for effective use beyond that range. The ambitious reader is encouraged to sift through the Huntsville tests (forgive me for not doing the legwork on this one) to find among them charts where I overlaid the resistance curves from a single test using both thermocouple and germanium thermometer-derived temperatures. I too tinkered with calibration efforts, but only in attempts to bring the curves together somewhere (anywhere) in the vicinity of 25 K. The primary difficulty was always inconsistent and explosive growth in the germanium thermometer errors at increasing temperatures. I would remind the reader that our previous application of the germanium thermometer had been measurements in the 5 to 10 K range, territory in which it performs admirably.
To explore the possibility of thermometry issues, I took the liberty of reading the third pass from the 25 November 1986 data (double “prime,” black, H=400 mV), the first indication of the supposed disappearance of the 75 K superconductivity. The data is shown here without any biases or scaling, conveniently included with the first two passes and charted on a logarithmic x-scale.
25 November 1986 sample #1b reconstruction with third pass added (double “prime,” black, H=400 mV). Logarithmic temperature scale and 5 K intervals. Inset includes additional points.
The points at or below 25 K in the third pass were estimated assuming a linear relationship between R and 1/T for the resistance thermometer.[358] Since offsets and scales for the curves corresponding to the points between 10 and 20 K were not noted with the original data, I presumed them to follow the trends of the other sets (e.g., transitioning from 90 Ω and 1 Ω/in to 100 Ω and 10 Ω/in). The vertical trend would have forced an adjustment in the y-offset on the final segment that I have assumed to be precisely one full vertical span[359] of the chart (1 mV) at the current scale of 0.1 mV/in. Independent of the precision of my assumptions, the general shape of the third curve is true to the original data; double-checking my work is certainly encouraged.
I will simply leave the above chart to the reader (while further noting that if the sample was measured with direct current, then the thermometer was also likely measured with that same method so vulnerable to biases) to discern whether the differences between the first two curves and the third are 1) evidence for sample properties having changed at corresponding temperatures or 2) evidence for the temperature readings having changed at corresponding sample conditions. Put another way, is the most plausible explanation for the results bad thermometry or world record high temperature superconductivity?
Interestingly, the tests on 25 November 1987 were not the only attention received by sample #1b. The adventurous reader who may have gone to the trouble to secure Exhibit #419 (from the ongoing Hor-Chu case) referenced earlier is also encouraged to examine results of any other tests on sample #1b during the period of roughly the final week of November. Incidentally, it should be noted that the composition of sample #1b is (not surprising given the number in the designator) precisely one of the two compositions originally explored by the Nobel-winning Bednorz-Müller team. The IBM duo reported only detecting superconductivity “with an onset around 35 K,” and this despite experimenting with a fairly wide range of processing temperatures and atmospheres.[360]
Returning to the “High Temperature Superconductivity” account, Chu’s final assessment on sample #1b is this:
In an attempt to show that the large R-drop at ~ 75 K was associated with a superconducting transition, we tried to examine the magnetic field effect on the R-drop. Unfortunately, due to some experimental difficulties, an unambiguous conclusion could not be drawn…[361]
While I would agree with the basic assessment, in my opinion the “ambiguity” of the test should have rendered it unworthy of publication in any forum. The arbitrary “bias adjustment” and accompanying claims of a nearly complete disappearance of resistance are indefensible, and had the results at least never appeared in print, it might have even been justifiable to excuse overlooking otherwise glaring indications of flawed thermometry. The absence of tick marks on the third and subsequent passes certainly suggest that those curves may have only been eye-balled and never charted, but such tests on the heels of efforts to calibrate a thermometer clearly ill-suited for the higher temperatures would mandate closer scrutiny before advertising claims of record high temperature superconductivity.
One final issue with Sample #1b that I noted more recently... Chu indicates that the drop was by a factor of 80, thus suggesting some residual resistance. The upward trend below the transition is certainly indicative of this (if we can somehow make sense of the negative values). Now observe in the first two curves that there is no second drop at 35 K despite some residual resistance. Did the new transition (and whatever phase was its cause) coincidentally appear just as the 75 K superconductor vanished... and on the third pass, no less? If we are to accept the thermometry, then the most "logical" explanation is that the 75 K phase morphed into the "Bednorz-Müller" 35 K phase (a transformation further "proven" by the nearly identical R-drop factors). But if the two phases are polymorphs (same composition but different structures) and we thus know the composition of the 75 K phase, should it continue to be so difficult to isolate and identify its elusively unstable structure?
Appendix B, Chu/Wu Videos from 1987
Very late in the finalization of this manuscript, I came across the website of Paul Grant,[362] a defender of Chu (see the August 1987 Science article) who has also done an amazing job archiving hard-to-find items relevant to the superconductor activities of 1987. His collection includes several videos of some interest.
The first video is of a presentation by Paul Chu at the infamous Spring 1987 American Physical Society Meeting[363] aka “The Woodstock of Physics,” an event whose excitement was driven in large part by the discovery documented herein. In reviewing the video, there were a couple of matters of note but nothing inconsistent with my previous observations. If Novel Superconductivity marked the births of the J-31 and Sample #1b legends, then this presentation would mark their conception. Sample #1b makes a brief appearance at the 3:50 mark. Not surprisingly, its bias is already removed, as it would seem unlikely that Chu would ever attempt to pass off a chart with “negative resistance.” Then at the 4:20 mark appears the most humorous part of the briefing. He starts, “Here I can also show you another compound which shows magnetic measurement starting 100 degrees [Kelvin].” At this point, Chu places the viewgraph on the projector, but turns it upside down, stating, “purposely turn it upside-down, going like this, starting from 100 degrees but unfortunately this signal disappears after a day or so.” Apparently, whoever originally charted the data believed that the original tests had the sample and background labels reversed, thus flipping the sign of the net signal and likely unraveling much of the mystery of J-31. At the 9:30 mark, Chu makes mention of “many occurrences” of unstable superconductivity at 240 K (recall the quote from Robert Cava in my Conclusion). If there is some reproducibility (“many occurrences”), how can the 240 K superconductor so defy isolation and identification? By Chu’s July 1987 “Superconductivity above 90 K” paper,[364] it was “not unforeseeable to take another few months to stabilize the superconducting phase with a Tc at 240 K.” As of this writing, it has been over 340 months.
At a minimum, the APS video indicates that Chu wasted little time finding every curve with an apparent transition at or near 70 K and weaving a dramatic story from them. Of course, not even a hint of how those tests supposedly influenced what happened in Huntsville (as implied) is ever offered.
The second video is a presentation by Chu at the Spring 1987 Materials Research Society Meeting in Anaheim, California.[365] The reader may recall that this is the gathering where Rustum Roy and Amar Bhalla interrogated Wu and Chu. Beginning at the 1:25 mark, Chu greatly emphasizes the supposed importance of the pressure studies. He then makes mention of signs of 70 K superconductivity (presumably either J-31 and/or Sample #1b), along with multiple references to atomic radius. At the 2:10 mark, he states, “When made as pure K2NiF4 [structure], the onset temperature always drops,” a notion that seems to suggest that any and all impurities accompanying that structure (20-40 K) always enhance the critical temperature. Of course, he is proposing that it was the intent of Y1.2Ba0.8CuO4 to make something other than the 2-1-4 structure, to which the intelligent reader would ask, if one is trying to make something different from 2-1-4, why keep mixing 2-1-4 (noting 1.2+0.8=2)? Again, we receive no details of how the “70 K observations” actually influenced activities in Huntsville, moving straight to “finally on January 29th.” The reader with some expertise in the field may also wish to listen to the Q&A at the end of the video.
The third video is a presentation by Wu at the same meeting.[366] I had hoped to find some mention about how the critical YBCO composition was actually formulated, especially since I had not yet confronted Wu by that time (an encounter to occur a few weeks later). However, given Chu’s presence at the same meeting and what has been told to me of the their relationship, it is unsurprising to me that Wu would avoid presenting anything that might collide with the evolving story coming out of Houston. For many years, with Chu on one flank and myself on the other, Wu continued to be reluctant to detail how the discovery came to pass, preferring only to proffer some limited information that he could at least marginally reconcile with both actual events and Chu’s dynamic stories.
The fourth video is a presentation by Chu at the June 1987 Berkeley Workshop[367] from which the aforementioned Novel Superconductivity paper originated. The 4:30 mark launches the usual “high pressure” pitch. At 5:33 are two bullets on the chart that can be tracked to Sample #1b and J-31 (correlate the dates). At 6:10 the Sample #1b resistivity is shown, again biased to avoid having to explain negative resistivity. At 6:20 Chu introduces the (my emphasis) “most convincing piece of evidence of superconductivity,… obtained on January 12th” (obviously J-31, see the chapter by the same name in Part II). Note that not only is the residual “bias” not yet removed, but there is an even more striking “adjustment” at 100 K (even after compensating for aspect). Apparently, however, someone must have deemed the adjustment too extreme (to the point of simply looking like a bump on the curve), as it was toned down by about half in the corresponding paper that was submitted to the meeting proceedings.[368] I would encourage the reader to review again the raw data behind J-31. Because it was originally charted in curve fragments that “wrap” at various points in the measurement (including about 105 K), I have copied segments of both the original sample test and the accompanying background test and fit them together here (consistent with the offsets documented by Houston) so that one can more easily view the original data in the 80-120 K range.
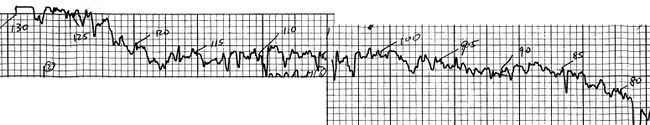
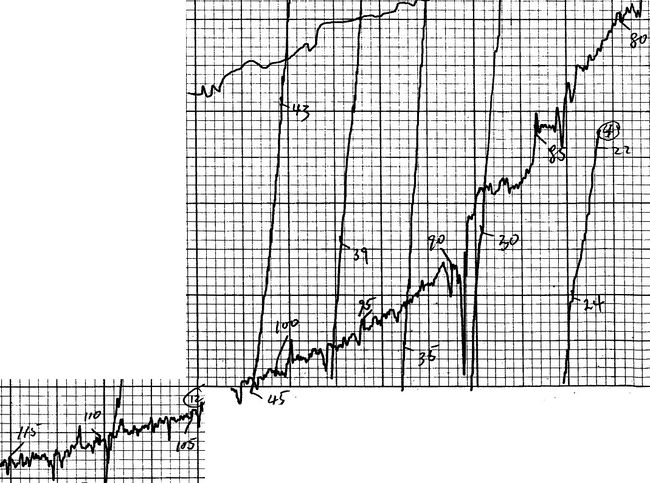
Snippets of the original J-31 data (Top: Sample+Background, Bottom: Background Only) from the 80-120 K range fit together to aid visibility (consistent with the offsets reported by the University of Houston).
First of all, it is important to recall that the overall shape of the net curve is dictated almost entirely by the background test (as first noticed by Hor[369]), whose smooth upward drift shows no indications of any discrete transition anywhere in the 80-120 K range. The sample (plus background) curve, on the other hand, would seem to show its most compelling feature (and that hindered by the overall noise level of the data) at around 118 K, but that portion of the curve was conveniently omitted in the published versions by truncating the plots at 120 K. Recall from the chapter on J-31 in Part II that the ideal net signal should be very near zero and flat at higher temperatures. Without the later arbitrary 3 mV bias removal, there is still the unexplained very large signal at high temperatures. Thus, looking for deviation in the net signal from zero for the purpose of identifying a transition onset is of no value. The next possibility is to seek the first deviation from a flat constant signal (assuming without any justification that the unexplained background is temperature-independent) or, if the bar is lowered further, the first deviation from a linear signal. At best (upon removal of the bump which, from a visual inspection of the original noisy data, should have been one of many), the net curve is at best asymptotically approaching level as the temperature increases[370] (i.e., no discrete transition at any particular temperature). Otherwise, there is nothing that could reasonably be labeled a “most convincing piece of evidence of superconductivity” around 100 K.
Again on the tails of J-31, Chu skips ahead in time and space (750 miles northeast to Huntsville) to “and then finally on January 29th” (7:15 mark). Fast-forwarding to 28:00, we see Chu introducing and then displaying a chart (28:30) showing a complete resistive transition at 225 K. Note the crowd reaction, with some standing up and one overheard asking “What’s the temperature?” Stable for a week, according to Chu. A paper subsequently appeared in Nature[371] documenting these never-again-seen observations, to which Chu has since been loath to draw attention.
At 29:50, Chu introduces a chart of apparent 130 K superconductivity in Gd-Ba-Cu-O (again, never since isolated or “stabilized”). For a closer look, the plot can be found in Chu’s Novel Superconductivity paper.[372] Personally, if I were to see an irreproducible result where the curves differ only in the horizontal (in this case, with smooth lateral displacement going all the way up to 200 K), I would assume a thermometry issue.[373] To my knowledge, the highest credible reported critical temperature in Gd-Ba-Cu-O is and continues to be about 93 K.
Appendix C, Chu’s 1990 Declaration
With the structure of my narrative in place and as I find additional items of interest, it becomes increasingly difficult to “work them into the flow.” Consequently, I include some of these new pieces as appendices with only brief commentary, leaving the more detailed analysis to the now well-informed reader.
Over the course of my investigations, the question of a Chu declaration in the original patent interference was often raised. A search of my legal files first yielded nothing, but I did eventually find that Chu, in his “Bannerot letter,” actually makes reference to such a declaration.[374] Chu’s comment kept me always on the watch. Then, upon yet another pass through my documents in search of other items, I spotted within a series of draft Meng declarations (ones including Chu’s hand edits to what are supposedly Meng’s words) a previously unnoticed rough of a Chu declaration. I will state clearly here that it is a draft and unsigned. Concerning the degree to which the words are attributable to Chu, I would encourage the reader to make comparisons of the hand-written edits to those in the various presentations (Urbana, Denver, St. Louis) included previously with this narrative.
I am providing a copy here[375] for the review of the reader who, having hopefully conquered the body of this narrative, is now well equipped to analyze the contents. Unlike the Meng testimony describing a 1 February discovery in Houston, the Chu declaration specifically acknowledges the 29 January discovery in Huntsville, explicitly states that the materials subsequently brought to Houston were immediately confirmed to be superconducting and then unequivocally affirms in paragraph 10 on page 4 that the Huntsville composition met all counts of the contested patent (which would include an oxygen ratio y in Y1.2Ba0.8CuOy between two and four as described in paragraph 5 on page 2, not the impossible value of one inferred by the USPTO). In other words, Chu declares the basis for the patent court’s final decision in the Huntsville-Houston interference as simply wrong.
The reader can explore on his own Chu’s discussions of Wu as his former student (with the obvious extrapolations to be inferred); blathering on high pressure; his conception of the “yttrium idea” in mid-December and immediate tasking of Ruling to make YBCO samples (resulting in one or two rounds of failures, depending upon which story is preferred); the supposedly “poor quality” of the Huntsville LSCO samples around the new year (see paragraph 14 and the edits to paragraph 6; if ours were poor, one can only wonder how the Houston-made YBCO failures should be characterized[376]); the initial shotgun patent application along with its atomic radii values; zero details on any Huntsville-Houston communications in the four weeks leading up to the discovery; Chu revealing his patent application to Wu on January 30th (counter to his story in the August ‘88 Science article); and waffling on whether or not the Huntsville team was actually tasked to examine YBCO (see deletions in paragraphs 13 and 14).
In the interest of some brevity, I will simply note that when I read Chu’s tale of his supposedly solo “conceptions” of the strontium and yttrium substitutions (paragraphs 5 and 6) and then consider the aforementioned papers by the Michel-Raveau group describing such substitutions (recall Chu’s desk calendar alluding to efforts to obtain papers from these very scientists a mere two weeks before his yttrium epiphany[377]), I cannot help but be reminded of Cava’s reference to “our [society’s] need to associate remarkable historical events with the great deeds of individuals rather than the collective efforts of many different people.”[378]
Lastly, having failed to resist delving into the thousands of pages of documentation in the ongoing (as of this writing) Hor-Chu case (thus, the next appendix), I located a “final” signed 1990 Chu declaration.[379] I do not anticipate the contents to be substantially different from the edited roughs, and I simply lack the energy for another pass.[380] I should note here that after substantial efforts I have been unable to find any evidence that this declaration was actually filed in the Huntsville-Houston interference, and I have been assured by attorney Steve Kelber that it was not (otherwise, Chu would have been deposed, according to Kelber). On the off chance it had been entered into the record, then its oversight by patent examiners and attorneys representing Huntsville alike was a very unfortunate misstep.[381]
Appendix D, Hor-Chu Case, Even More Documents
I must apologize to the reader up front for this appendix. The quality of my writing, if there ever was any previously, will now take a southward turn. I am simply trying to relay the information at this point and am too tired to care anymore how stylish my words may be. My theme for this section is taken from some words I was recently told originated with the editor of the Harvard Law Review (possibly a paraphrase): “Only a very few have bemoaned the passage of logical reasoning from the body and practice of our law.”
The federal court handling the ongoing Hor vs. Chu case (Civil Action No. 4:08-cv-3584) recently made public a substantial number of items of interest. I had been prompted to go looking upon having recently been provided a copy of the “Findings of Fact and Conclusions of Law” (in my understanding, the final ruling) from the case. I have linked that initial document here.[382] Upon visiting one of the websites that archives the official court documents, I discovered, in addition to the various legal filings and other testimony, references to hundreds of “supporting” exhibits.[383] Despite my waning energy in this undertaking (not to mention a family that is awaiting my return from this Dantean journey) I have been compelled (for reasons about which I would rather not elaborate) to yet again at least briefly cross the Acheron.
As I understand the case, Hor is making two claims. His initial claim is that he, not Chu, was the first to conceive of substituting yttrium in the copper oxide superconductors and that he relayed this “idea” to Wu on or about the first of January 1987. The second claim, about which I have nothing to offer, concerns the substitution of other rare earth elements for yttrium in the YBCO (“123”) superconductor.
Hor argues that his early January discussions with Wu prompted the eventual discovery in Huntsville (we will look at this more closely soon). In the original patent interference, any reliance on those discussions appears to have been the Houston “backup plan” in the event the Patent and Trademark Office (PTO) concluded that the discovery (the physical “reduction to practice”) occurred in Huntsville. The PTO instead concluded that the Huntsville team made some impossible superconducting sub-oxide, contrary to physics, chemistry, everything Chu has ever written about the discovery, and (most unfortunately) the scope of the patent count being contested.
Thus the first irony is that, while Chu won the interference based upon a legal determination that the physical reduction to practice did not occur in Huntsville, Hor, Meng, and Chu are now fighting over who said the word yttrium to Wu. The second irony is that “trying yttrium” in the copper oxide materials was a universally obvious measure to consider, demonstrated ad nauseum in Part II. So, it is of little surprise that no fewer than five persons between the Huntsville and Houston teams will stake some claim to it, including Wu, myself (with some credit due Er-Rakho et al.), Chu, Hor, and (as will be noted by the federal court judge) Meng. The third irony is that “trying yttrium” in the copper oxide materials does not universally yield a superconductor, as three different teams (Bellcore, Tokyo, and Houston[384]) reported only failures prior to the 29 January discovery in Huntsville, failures using even the right combination of elements but doomed by unfavorable ratios and processing temperatures (one can only imagine how many others tried yttrium but, for obvious reasons, did not report it, much less claim to have been responsible for the discovery). The bottom line is that any debate over who might have been the first to have “conceived” of the “yttrium idea” is nothing less than asinine. Towards the end of more firmly validating my characterization, let us dig just a little bit deeper into the Hor-Chu case, starting with the court’s observations as documented in their “Findings of Fact.”[385]
First, it is intriguing to note Chu, Hor, and Meng have now fully abandoned the 1 February discovery in Houston that was the basis for their university’s position in the interference with Huntsville and are presently (again, the case is ongoing as of this writing) vying for a patent in Chu’s possession by debating an issue (the yttrium utterance to Wu) whose presumed relevance alone (independent of its resolution) would invalidate the conclusion (that the Huntsville team was not the first to make the YBCO superconductor) by which the contested patent was awarded to Chu in the first place.
I furthermore find it fascinating in a case where (again disregarding the issue of the other rare earths) everything necks down to a single utterance of proven insignificance and for which there are no notes or recordings, that the two parties can somehow collectively believe it possible for there to exist thousands of pages of relevant “supporting” evidence, significant quantities of which seem to be devoted to attempts to demonstrate their respective levels of intelligence.[386]
Second, now 28 years later, we still have no claims, much less evidence, that any additional information was communicated from Houston to Huntsville between the supposed yttrium utterance and the 29 January discovery, information like a specific composition (elements and ratios) or processing, details hardly irrelevant given the acknowledged failures with yttrium by Houston over the course of January. On that topic, one may note this statement on page 16 (my emphasis):
…on January 29, Dr. Wu reported observing superconductivity at 77 K in a mixed-phase compound in which Yttrium was substituted for Lanthanum. (Doc. No. 184 at 143.) In reporting this development, Dr. Wu told Dr. Hor that he had accomplished “what we discussed previously,” referring to the early January meeting in Houston. (Pl. Trial Ex. 49 at 808.) Because Dr. Chu was unaware of what had been discussed in that meeting, he asked Dr. Hor to write down the formulas he had discussed with Dr. Wu. Those formulas included the Yttrium and Scandium substitutions for Lanthanum. Though Ms. Meng tried to duplicate the result with Yttrium that evening, she was unsuccessful. (Doc. No. 1:143, 150; 2:138; 3:16-17; 6:105-109; P. Ex. 21 (H-50).)
Of course, in Part II, I have already hammered the 29 (actually 30) January list of compositions and the supposed cramming of fabrication and testing of those samples into a single evening, so I will not belabor those points. I also noted in Part II that the patent court had greatly stretched the definition of inventor when, as of the evening of the 29th of January 1987, the discovery had already occurred, yet the University of Houston is still failing with yttrium (how does an invention precede one’s sufficient knowledge to achieve it?). However, I was a bit facetious given that the patent court had never had the opportunity to review Chu’s later accounts. In the Hor-Chu case, however, the now Federal District Court acknowledges that, in an apparent consensus of the Houston accounts, that the team still did not possess the knowledge to make an yttrium-based superconductor even after the time at which the discovery was conceded to have occurred. Given that I have so little understanding of legal matters, I cannot help but speculate whether or not the current civil case system permits a judgment for “none of the above.”
Third, footnote 11 (page 11, section on Plaintiff’s version) has an interesting analysis of the early January conversation:
How Dr. Wu learned of the Yttrium substitution is the topic of some disagreement. At trial, Dr. Chu testified that he told Dr. Wu of his conception of the Yttrium substitution in a telephone call in December 1986. (Doc. No. 190 at 41-43.) His sworn declaration in the Wu interference indicated otherwise. (Pl. Trial Ex. 63.) There, Dr. Chu explained that he did not discuss the Yttrium substitution with Dr. Wu prior to January 29, 1987, but that Dr. Hor or Ms. Meng likely informed Dr. Wu of the idea sometime between December 27, 1986 and January 4, 1987. (Pl. Trial Ex. 63 at 6; Doc. No. 190 at 55-59.)
I cannot help but sense a tinge of sarcasm here when the word disagreement is used to describe a conflict between Chu and himself. Nevertheless, it is fascinating that the court surmises from Chu’s declaration[387] that Hor or Meng only likely informed Wu of the idea during the New Year’s trip to Houston. It seems remarkable that what Chu so recently described as his “heavy personal involvement”[388] is now reduced to his assumption of a “likely” conversation. I would personally be interested in knowing how one determined it to be likely, much less relevant.
Fourth, across pages 12, 14, and 21, both parties confirm a 12 January date for the yttrium oxide order. Of course, the court fails to note that Meng’s “perjury affidavit” reported the yttrium oxide as arriving the next day (13 January), consistent with Chu’s response (page 14) that yttrium be ordered “soon and quick” and thus leaving as a mystery why, with such a supposed great sense of urgency, the first yttrium samples acknowledged to have been made in Houston do not come until the very end of the month. As will be seen shortly, the timelines offered in the Hor-Chu case pass seamlessly from the early January conversation straight to the 29 January discovery, conveniently leaving the impression of a month-long effort with yttrium at its center (see Part I for a fill-in of the intervening details so glibly glossed over by my Houston colleagues, including a more accurate description of the focus, or lack thereof, on yttrium upon our return to Huntsville).
Fifth, the court reiterates that Chu “conceived the Yttrium substitution on December 18” (page 14), a week and a half after I first read the 1981 Er-Rakho et al. paper documenting an yttrium-for-lanthanum substitution in La-Ba-Cu-O, and even though by his accounts he needed yttrium oxide “soon and quick,” he does not ask Meng to order it until 11 January (page 14) – three and a half weeks later – despite his supposed knowledge as indicated in his largely “past tense” December desk calendar that “yttrium has to work.”[389]
Finally, the courts note that the two parties “agree” that Chu asked Hor to write down the compositions supposedly related to Wu by Hor at the early January meeting (pages 16 and 17). Page 16 describes those compositions as including both yttrium and scandium before describing Meng’s mad-dash evening where she failed to achieve the results seen in Huntsville. Let us turn our attention now to the documents representing the individual testimonies, proceeding from junior-most to senior-most, where we will examine, among other topics, the question of the supposed early January “list” of compositions.
In Hor’s “Corrected Brief” from April 2012,[390] the page marked 4 describes the dramatic moment where, in the early January discussion with Wu, Hor pulls out his periodic table and “at that point, conceive[s] of the idea of replacing the element Lanthanum (La+++) with the iso-valent element Yttrium (Y+++) ion.” The next sentence states that this very same idea that failed to produce superconductors at Bellcore, Tokyo, and Houston (my emphasis) “resulted in the creation of a YBCO (Yttrium-Barium-Copper-Oxygen) compound (made initially by Wu) that exhibited superconductivity above 77°K.” The document then notes that Meng’s order for yttrium oxide could not be placed until January 12th because of UH’s winter break, after which it describes how Hor and Meng “ask Wu to… begin work on Yttrium substitution” (apparently entreaties from both were insufficient to light a fire under Wu as no one in Huntsville lifted a finger to do so until late January).
Page 5 describes Meng’s supposed suggestion for Wu to “get Yttrium oxide from ‘NASA in Alabama so we can start the work soon,’” betraying her apparently psychic knowledge that there was none in any of the UAH chemistry stockrooms. Concerning the question of whether or not such a search might have saved any time, I have exchanged several recent emails with NASA information personnel and have yet to confirm that Marshall Space Flight Center’s 170 buildings (many of them enormously large) have ever hosted a chemical stockroom. I would further point out that NASA is a government facility and so becomes almost as sleepy a place over the holiday break as a typical university. The document then jumps directly from the early January meeting to the 29 January discussion, as if not a single activity in Huntsville during the intervening weeks could possibly be of relevance.
Page 6 describes Chu, at some unspecified point after the Hor-Wu meeting, supposedly asking Hor to write down the formulas that were discussed. As I noted above, the courts discerned that this event took place on the evening of the 29th of January, consistent with what is described as those formulas being represented by the list appearing on the page marked 29 January[391] from Meng’s notes. I suppose one could make the case for deferring to Hor for the precise details of the early January meeting given that he demonstrates his remarkable memory here by precisely regurgitating off the top of his head 25 different chemical formulas four weeks after the supposed discussion (except that we learn later that only some of the compositions were his and that they somehow became interleaved with others he did not claim).
Page 6 then describes Wu’s 30 January arrival in Houston with a sample of composition Y1.2Ba0.8CuO4, thus seconding Chu’s refutation of the USPTO’s conclusion that the Huntsville team had instead made an oxygen deficient sample. Hor summarizes his effective rebuttal of the PTO’s decision this way:
Measurement of the sample showed reproducible Tc above 77K. Hor measured the Meissner effect which indicated the resistivity transition observed by Wu was a genuine superconducting transition, and that a superconductor with a Tc above liquid nitrogen temperature existed in Y1.2Ba0.8CuO4.
While my plan had been to address the documents chronologically, this next one slipped through the cracks, and in the interest of not having to try to rework the continuity of this appendix, I leave it here.
We now back up a few years to Hor’s 2009 deposition. On page 48, the attorney questioning Hor asks about Wu’s visit to Houston “near the end of 1986” (the reader might recall that I also was on that road trip, as confirmed in various accounts by Chu). Hor rambles a couple of pages about “mimicking pressure.” On page 51 he describes pulling out his periodic table (as if that were somehow an uncommon occurrence during that period, I suppose; by late December, I had the southwest corner of the periodic table memorized and can still, almost 30 years later recall at times many of the atomic weights of the elements of interest). On page 53, Hor states:
…if you look at lanthanum, okay, one on top of is yttrium, so if you want to choose an element to replace lanthanum, yttrium is the next choice.
He seems to be suggesting (correctly) that basic high school chemistry points the way to the next candidate which, combined with the pressure twaddle (and, to a lesser degree, the dangers of intense radioactivity), spared us from moving below lanthanum to actinium instead.
The attorney next asks if lutetium was discussed. He first states that he does not recall, and then remarks, “That’s later when it shows up.” However, the reader may note that lutetium accounts for eight (numbers 5-8, 18-21) of the twenty-five compositions on the “29” January list.[392] The discussion then proceeds with:
Q:During the meeting, did you actually write down any formulas then?
A:No.
Q:In other words, what you presented was the concept of an yttrium-barium-copper oxide composition but without regard to formulation?
A:No, because this is a so-called exploratory type of research, we are going to expand the composition, so far as I’m concerned, I don’t have to write down any specific formula because as you can see on January 29th the formula I have written down, which you have this composition go through different regions because you want to – you want to cover all the grounds of composition. You are not going to pinpoint to one particular composition because that’s brand new. That’s brand-new exploratory type of research.
Once again (my apologies) I must invoke the failures by Tokyo, Bellcore, and Houston with the right elements but the wrong “specific formulas.”
Here, by Hor’s account, there was nothing offered the Huntsville team except “try yttrium.” Meanwhile, the Huntsville team was left with the “trivial” task of deducing which elements to combine with yttrium, in what ratios, and at what processing temperatures. While the “29” January list might imply a relatively short range of possibilities, combinatorial mathematics would suggest otherwise (see, for example, my examination of the Chu “shotgun” patent applications). I would also encourage the reader to reexamine the page marked H476 from Meng’s notes[393] for a list of several one-off failures made in Houston, elemental combinations apparently dropped without any attempts (certainly not immediately or urgently) to “cover all the grounds of composition.” With all due respect to Hor, it is the needle in the yttrium haystack that is the hard part.
Continuing, on page 60, Hor is asked why the focus on yttrium. He responds, “It’s right above lanthanum [on the periodic table],” thus reemphasizing the painfully obvious nature of yttrium as a candidate. On page 62, Hor confirms receipt of the yttrium oxide in “mid January,” not late January as suggested by so many of the Chu accounts. Page 65 narrows the date to the 14th as I previously demonstrated.
Remarkably, on page 71 there is a brief discussion of the mid-January (“January 13th through January 15th”) yttrium failures (finally the existence of those tests is acknowledged). The attorney asks about a composition “showing an yttrium of .9 and a strontium of .1” (sample YS-1). In answer to the question of whether or not those samples were “actually made,” Hor notes, “if the composition has been calculated,” referring to the raw material weights needed for processing, “it should be made.” He then explains why the work was quickly terminated, “At that time, we are starting to do this but we stop because of this report of our sample become non-superconductive,” which would make one wonder when or if Houston would have ever returned to yttrium in the absence of the discovery in Huntsville. The fact that investigations of particular combinations of elements are terminated after only single specific formulas of each are made and tested also raises a number of questions concerning how Hor’s “exploratory type of research [where] you want to cover all the grounds of composition” actually works.
On page 73, Hor is asked, “Were more samples made at the university [Houston] that included yttrium-barium-copper oxide?” Hor answers (the entire quote is worthy of emphasis), “Yes, after Dr. Wu reveal… his composition, yes.” Finally, we have the truth on what prompted the late-January flurry of Houston activity with yttrium,[394] and Meng’s list makes sense as the locus of new candidates judged as neighboring the observations in Huntsville – varying ratios in the Y1.2Ba0.8CuO4 composition, a lutetium replacement for yttrium, and a lead replacement for mercury. As Hor describes, successes are more likely than failures to become the focal points for more exploration.
On page 74, the attorney asks, “After Dr. Wu left, do you know what work Dr. Wu continued to do?” The response: “No.” Recall that none of the members of the Houston team have ever offered any meaningful details on the other “exploratory” work done in Huntsville in the weeks leading up to the discovery. Chu, via Hazen, falsely suggested that we were slowly zeroing in on the right yttrium composition. In short, for the month of January, Houston claims only the critical Y1.2Ba0.8CuO4 composition and by Hazen the mythical variants leading up to it. Now we learn here that they (or Hor, at a minimum) had no knowledge of anything we did after our return to Huntsville.[395] Given that our first YBCO sample was fabricated on January 28th and tested the following day, does it not seem all too convenient for the Houston team to claim responsibility for our activities during only that two-day window out of everything we did over the course of 1987?
Page 84 starts a discussion about the “Y to Yb” alterations that appear in Meng’s notes shortly after the discovery.[396] Hor expresses concerns that the pages have been altered. For some background, I would direct the reader to the main body of this text for a lengthy discussion of topics related to the “Yb or Not Yb” question.[397] On page 86, Hor directs the attorney’s attention to “Page 51,” presumably the page marked H51 from Meng’s notes.[398] He describes how the symbol for yttrium has been altered to indicate ytterbium (again, as explained in the main body of this manuscript). Despite being among those present when the ytterbium typo was discussed for inclusion in the original YBCO paper, Hor states, “This is something which I cannot imagine.” Hor requests a review of the original. I can assure him that the small b’s were almost certainly added during the time period the ytterbium typo strategy was being formulated prior to the submission of the paper. He will find no copies without them (legitimate ones, anyway).
On page 89, Hor states that he had previously misidentified these samples as his work (calling it February work but on the next page making reference to January 30 and page 51), but then states, “After I had a chance to look into it the formula, that formula doesn’t make any sense to me and is definitely not my idea.” He then describes the formulas as “technically incorrect… scientifically, simply wrong.”
Of course, given that I had discussed with him these compositions as early as 2006 and explained that I could precisely recreate the three-digit precise formulas (again, see the main body of this work), Hor had no choice but to retract his previous assertion. He then went on to play up their “technical incorrectness” (the recurring incompetence-of-others theme). Despite whatever the degree to which they were “scientifically wrong,” they were still closely related to the same ideas that yielded the discovery that precipitated this insanity.
On page 95, Hor gives a very nice discussion of how Chu’s atomic sizes are inappropriate for use in examining ionic compounds (see the main body for more on this).[399] On pages 100 and 101, Hor correctly indicates the intent (my intent, to be more precise) with Y1.2Ba0.8CuO4 was to make a compound of the “2-1-4” structure, contrary to Chu’s ridiculous stories of trying to stabilize some other structure during that period (again, see the main body of this work for more on this). Page 144 has some more blathering on how one can simulate the effects of pressure by “squeezing” smaller ions in the place of larger ones in the crystal structure.
Page 146 returns to a discussion of the early January Wu meeting:
Q:And on that day when you made the suggestion to Dr. Wu, you didn’t present any formulas, do you?
A:No.
Q:You didn’t present any experiments, did you?
A:By the formula – the experiment will be in 2-1-4.
Q:Just answer me.
A:Right.
Q:On that day you said it must be yttrium, you didn’t present any details or formulas or anything, did you?
A:No.
Clearly the Huntsville team was left with the simple task of trying to solve the trivial combinatoric[400] issues of the other elements (barium, strontium, calcium, mercury, lead?), ratios (an even bigger problem), and processing (air? oxygen? vacuum? 1100 °C? more? less? Luck helps here as previously discussed).
On page 153 in reference to the “29” January list, we learn that the yttrium and scandium ideas were supposedly Hor’s ideas while Meng added lutetium compositions to the list. Hor even identifies by number (1-4, 9, 11-17, and 22-26) all of the yttrium and scandium compositions, after which he reiterates (pages 154 and 155) that no precise formulas were actually communicated to Wu. When questioned about scandium, he notes that the “idea” of scandium was self-evident, stating (my emphases):
Q:All right. Where did the idea of scandium come from?
A:They’re in the same group [of chemical elements].
Q:What group?
A:If you look at the lanthanum, above [on the periodic table] lanthanum is the yttrium. Above [on the periodic table] yttrium is the scandium, so anyone with the [basic chemistry] knowledge to try to replace the elements automatically, you know that in the same column, they all can be replaced.
I could not have described it any better myself, but one can only wonder how scandium was “automatic” while yttrium was a stroke of brilliance. On pages 154 and 155, we learn that the suggestion of scandium was first made on 29 January, and thus the parts of the page supposedly traceable to the early January Hor-Wu meeting are now limited to only the yttrium formulas.
The attorney then moves forward one page in the lab record (from 1129 to 1130; in my records, from the single page of Exhibit G to the first page of Exhibit H[401]). He specifically asks about the formula “lanthanum, yttrium, barium, copper, oxygen,” and Hor confirms that it is not his. That composition is among the first of the 3-digit precise composition described in agonizing detail in the Part I. As noted earlier, I had told Hor as early as 2006 that I could reproduce these compositions, leaving him no choice but to disavow them. He declines to offer his knowledge that they are mine (a proverbial can of worms, I am sure), but does remind the attorney of the Y-to-Yb alterations, as if to tie the Yb trickery to my formulas.
To summarize Hor’s account, he offers only repeated confirmations of the complete account I have provided in the main body of this work. We now move on to Ruling Meng’s story as offered in the Hor-Chu case.
For what it is worth, I have been far too weary to read all of the drivel in this case. My strategy has mainly been to start with searches for the word “yttrium” and proceed from there (“exploratory type of research,” one might say). The more energetic reader is certainly encouraged to peruse all of the material. I have offered it up with little concern that anything will be found contradictory to my story. So far, as with Hor, I seem only to find information that further drives home my main points.
With Meng, we will start (and hopefully finish) with her deposition from 2010.[402] Most information presented across the 634 pages of testimony appears to be either irrelevant (“tricky processing” discussions, for example) or simply redundant with what I have already covered. Thus, I will focus only on some of the highlights.
On page 102, Meng reaffirms Hor’s assertion that no specific formulas were communicated to Wu in the early January meeting. Thus, we can conclude that only the necessary and sufficient information to fail with yttrium was provided.
On page 114, Meng describes her supposed conversation with Wu about getting yttrium from “NASA.” It is agonizingly difficult not to use some very unprofessional language in my characterization of her story. On page 117, Meng now confirms receipt of the yttrium oxide on the 14th of January, making me most happy to have finally settled that question.
On page 419, Meng breaches a topic that I will leave to the reader to interpret. Part of that discussion extends into page 420 where she suggests that she “must be the co-inventor” by virtue of her qualifications. I have no doubt that by actually reading the deposition in its entirety, I could write another book – yet another exercise I must leave to the reader.
Moving on to Chu’s accounts, I offer his brief here.[403] The reader can peruse pages marked 6 to 9 (the meat of the story). There are no dragons discussed that I have not already slain.
Next is Chu’s reply to Hor.[404] Pages 3 and 4 indicate that Meng could have ordered yttrium oxide as early as January 5th. Given that Wu and I arrived back in Huntsville late on January 4th, a hypothetical next-day Houston order on the 5th arriving even two days later on the 7th and allowing 24 hours for sample processing (no evening cram session) could have had Houston testing yttrium samples as early as the 8th, one day before we in Huntsville received our new LHe shipment and resumed our testing. So much for saving two weeks of time. On page 4, Chu reiterates (following Meng and Hor) that, at most, only elements were discussed in the early January meeting, not formulas, thus establishing a consensus that the sufficient and necessary information to actually achieve the discovery was never communicated to Huntsville by anyone in Houston. Chu caps his comments on the early January meeting with this: “Hor fails to explain how the suggestion of one element turned into a series of formulations.” An excellent point.
Appendix E, “The Chu Effect”
The particularly bold reader might be game for attempting to verify (or invalidate) “the Chu Effect.” While Chu’s superiors seem to limit the definition of “the Chu Effect” to the effects of high hydrostatic pressure on the critical temperature of superconductors in general, Chu and his Houston team members extend the definition first to a supposed negative correlation between critical temperatures in the copper oxide superconductors and certain unspecified interatomic distances. The extension further suggests that this correlation extends across diverse superconducting phases and that the effects of pressure on said unspecified interatomic distances can be chemically simulated using the appropriate atomic substitutions. Because the only common elements between the high temperature superconductors of interest are copper and oxygen and given the dearth of technical details on “the Chu Effect,” this section will proceed under the assumption that the interatomic distances of interest are represented by the Cu-O bond lengths.
As previously noted, the first historical experiments of the effects of pressure on the critical temperatures of superconductors were conducted by Sizoo and Onnes in 1925,[405] suggesting that, at a minimum, “the Chu Effect” designator should be confined to the “extensions” promoted by Chu and his team. Thus, this appendix will focus on the supposed critical temperature/interatomic distance correlation (positive or negative) and, to a lesser degree, the idea of simulating pressure effects on these distances with chemical substitutions.
The CRC Handbook of Chemistry and Physics maintains a fairly up-to-date table of the more prominent high-temperature superconductors (“Structural Parameters and Approximate Tc Values of High-Temperature Superconductors”) that includes their unit cell parameters along with the critical temperatures. With just the numbers provided, one can obtain a relatively simple quick estimate of the possible correlation between Cu-O bond lengths and critical temperatures. Starting with the CRC list, the examination is first restricted to copper oxides, thus eliminating Ba1−xKxBiO3 and the C60 fullerenes based upon doping with Rb, Cs, and/or K. Next, the focus is further restricted to the more prominent hole-doped copper oxides, thus excluding the n-type materials Nd2−xCexCuO4 (and variants with La and Pr) and the “infinite layer compounds” Ca1-xSrxCuO2 and Sr1-xNdxCuO2.
Given the nature of the copper oxide superconductors, the structural parameters identified in the table by “a” and “b” usually (more on the qualification shortly) represent twice the in-plane Cu-O bond lengths for the corresponding axes. This is generally true for any of the compounds where the “a” and “b” parameters fall in the approximate range of about 3.7 to 4.0 Angstroms. Occasionally values in the range of about 5.2 to 5.7 Angstroms will appear, indicating a larger unit cell such that the in-plane Cu-O bond length is given by:
- a / ( 2 sqrt( 2 ) ) if no “b” value is given (tetragonals)
- sqrt( a2 + b2 ) / 4 if “a” and “b” are given (orthorhombics)
Given this guidance, in-plane Cu-O bond lengths can be estimated for each of the approximately 40 (as of this writing) materials. Calculated values should generally fall in the range of 1.8 to 2.0 Angstroms. At this point, one can note a significant positive correlation between critical temperatures and in-plane Cu-O bond lengths. My quick estimate yields a Pearson correlation coefficient of +0.42.
Thus, at first glance, “the Chu Effect” already appears to be in trouble. For the reader determined to give it every benefit of the doubt, I offer the following caveats. First of all, in several of the copper oxide superconductors, the “in-plane” oxygen ions are perturbed slightly from the plane defined by the associated copper ions (i.e., along the crystal “c-axis”). To make more precise calculations of the in-plane distances, one is directed to the more detailed atomic position data available at sites such as the Crystallography Open Database (http://www.crystallography.net/). A primer on crystallography is beyond the scope of this work. I will note however that the displacements are not nearly large enough (usually less than 1%), frequent enough, and sufficiently focused among the lower critical temperature materials to effect a significant adjustment to the previously-estimated correlation coefficient, much less a reversal. The reader is certainly welcome to double-check my assertion.
The second qualification concerns the other Cu-O bonds within the copper oxide superconductors, in particular the bond between the copper ions and the so-called apical oxygen ions, those aligned with the copper ions along the longer c-axis of the structures (a study here of the Jahn-Teller Effect is intriguing). Again, the reader would need to turn to data sources such as the Crystallography Open Database to obtain the atomic position data needed to perform the analysis. In a given copper oxide superconductor, there may be multiple different bond lengths between copper ions and apical oxygen ions (complicating the analysis), and I will note here that the shortest such distances in YBa2Cu3O7 (our superconductor of greatest interest in this narrative) are unusually short, which would seem to help substantiate “the Chu Effect.” However, my cursory examination of several of the other copper oxide superconductors suggests that the short bonds in YBa2Cu3O7 do not mark the beginnings of any trend. Furthermore, the short bonds in YBa2Cu3O7 are adjacent to the very large Ba2+ ions, not the relatively small Y3+ ions that supposedly effected the change, thus conflicting with the key premise of “the Chu Effect,” that smaller ions reduce the relevant (whichever those might be) interatomic distances. Kamimura et al.[406] [407] indicate that in La2-xSrxCuO4, the distance between the copper ions and the apical oxygens decreases when La3+ is replaced by Sr2+, the latter being larger than or at least no smaller than the former (depending upon the source used for ionic radii). Kamimura further notes that it is the valence and not the size of the substituted ions that precipitates the shift in the position of the oxygen. That lower valence states generally correspond to larger ionic radii suggests that larger ions might actually tend to produce the smallest distances between the copper ions and the apical oxygen, consistent with the unusually small copper-to-apical-oxygen distances in YBa2Cu3O7 in closest proximity to the very large Ba2+ ions.
About the Author
Jim Ashburn was born in Huntsville, Alabama in 1964 as Huntsville’s Marshall Space Flight Center was approaching full speed on the Apollo program. Growing up among real rocket scientists, he became the quintessential geek. Pimply, greasy-haired, and painfully shy, he graduated valedictorian in a class of 300+ from Huntsville High School in 1982 – National Merit Finalist, winner of all manner of regional awards in everything from geometry to biology to poetry, composite scores on standardized tests routinely at the 99th percentile. In short, a resume many nerds would envy.
Clueless about what he wanted to do in life, his lack of passion was a great handicap in writing scholarship application essays. Vanderbilt declined to pay his way, after which he accepted his next option, a free ride to UAH in his hometown. One year in, he changed majors from pre-med to physics.[408] At the start of his junior year he took a 200 level mechanics class under M. K. Wu. Wu had recently joined the UAH Physics Department as a junior professor and soon hired Jim to work in his lab. For minimum wage, Jim assisted in the fabrication and testing of immiscible alloys in microgravity environments, namely NASA’s drop tube, drop tower, and KC-135 aircraft.
Despite his late start in physics, he still graduated in four years, a perfect GPA tarnished by two B’s the one semester he took his heaviest load and experimented briefly with skipping class to play disc golf. Since the only less-than-perfect grades were in classes in his major, he proudly claims physics as his worst subject.
Hoping to eventually fly with his lab’s furnace on the “Vomit Comet,” he remained at UAH for graduate school and continued to work in the superconductivity lab. After the very trying experience described in this narrative, he somehow managed to complete his dissertation without killing anyone (including himself) and graduate with his Ph. D. in physics in December of 1990 (3.97 composite college GPA, for whatever that is worth), after which he politely declined a post-doctoral opportunity at the Los Alamos National Laboratory,[409] abandoned his plans for a career in academia, and took a job in the defense industry in Huntsville (where he still finds several opportunities to do innovative work). He married Greta K. Branson of Nashville, Tennessee in 1991. Together they have five children, Christopher, Matthew, and triplets Kathryn, William, and Laura Grace, who know the superconductor discovery as “the stuff Daddy doesn’t like to talk about very much.”
References
- ↑ A note concerning temperature scales in this narrative: Because it was our convention at the time, temperatures at which samples are processed by heating will be reported in degrees Celsius, whereas temperatures associated with low temperature measurements will be reported in Kelvins.
- ↑ “YBCO” is commonly-used shorthand for materials consisting of the elements yttrium, barium, copper, and oxygen. Multi-phased materials may be specifically referenced by their composite compositions or, within their context, alternately identified as yttrium barium copper oxide, Y-Ba-Cu-O, or YBCO. The pure-phase 90 K superconductor YBa2Cu3O7-y may be referenced by its specific chemical formula, often approximated as YBa2Cu3O7 (since y is ideally small), or simply called YBCO, Y123, or (in a context where the first element is understood) 123 or 1-2-3.
- ↑ R. J. Cava, “Oxide Superconductors,” J. Am. Ceram. Soc. 83.1 (2000): 5-28.
- ↑ Often informally abbreviated as "liquid nitrogen superconductor," not to suggest that the superconductor is itself composed of liquid nitrogen.
- ↑ For someone who grew up in the ‘60s and ‘70s in “The Rocket City,” flying on the Vomit Comet was my consolation prize for being on the tall side for a prospective 1980s shuttle astronaut. I only discovered later that the range is more generous for mission specialists.
- ↑ C. J. went on from UAH to complete his Ph. D. at the University of Minnesota. He has since specialized in magnetic materials and, by my most recent records, currently works at Headway Technologies in the San Jose area.
- ↑ Bednorz and Müller subsequently won the 1987 Nobel Prize in Physics for this achievement. J. G. Bednorz and K. A. Müller, “Possible High Tc Superconductivity in the Ba-La-Cu-O System,” Z. Phys. B, Condensed Matter 64 (1986): 189-193.
- ↑ As a point of reference, about 75 years of superconductivity research had brought the temperature at which superconductivity was known to occur (the “critical temperature” or Tc) from just above the temperature of liquid helium (4.2 K in the metal mercury) to a high of 23.2 K (Nb3Ge). Extrapolating at that pace (and discounting the fact that there had been no progress towards higher temperatures from 1973 to 1986), the prospects for superconductivity at room temperature (~294 K) or at the perhaps more reachable boiling point of liquid nitrogen (77.35 K) would be expected to occur around 3050 and 2200 AD, respectively. To whatever degree Star Trek is prophetic, this would suggest that superconductivity above the boiling point of liquid nitrogen would only slightly predate faster-than-light travel.
- ↑ In the physical sciences, the term “phase” refers to a material whose chemical composition and physical properties are relatively uniform. For crystalline solids, a “phase” is usually equated with a particular crystal lattice, atoms of certain elements in a specific ordered spatial arrangement. Vacancies, defects, and “solid solutions” (where atoms of two or more different elements may substitute for one another somewhat randomly at a particular site within the lattice) sometimes allow for a range of compositions associated with a given “phase.”
- ↑ Located on the Army’s Redstone Arsenal bordering Huntsville, about an eight mile drive from the UAH Science Building (now Wilson Hall).
- ↑ Many were in French, for which years of German classes were no help, but I was still able to glean much useful information from chemical formulas, temperatures, figures, and the like, not to mention a few of the French words similar to their English counterparts.
- ↑ See, for example, page 9 of “Amended Memorandum and Order Entering Findings of Fact and Conclusions of Law” from Civil Action No. 4:08-cv-3584, Pei-Herng Hor vs. Ching-Wu “Paul” Chu. This document will be covered more extensively in Appendix D where a copy will be linked. It will note, “Neither Dr. Hor nor Ms. Meng dispute that it was Dr. Chu who conceived of replacing Barium with Strontium.” Presumably, Chu related to Hor and Meng the “idea” of the strontium substitution before relating the paper from which it was drawn.
- ↑ Prompted by the French papers, several other groups will test the strontium compound that December (see the analysis of the Hazen book later in this manuscript).
- ↑ Similar to the strontium substitution, several groups will also attempt an yttrium substitution. However, unlike the strontium substitution, all but one will encounter unfavorable results.
- ↑ In this context, “metallic” means conductivity that increases (equivalently, resistivity that decreases) with decreasing temperature.
- ↑ Including Chu, as will be seen in an August 1988 Science article to be covered later.
- ↑ The term “copper oxide” is frequently-used shorthand for this class of superconductors that are oxides of copper and other metals.
- ↑ A professor at George Mason University as of this writing (December 2014).
- ↑ Elmer Anderson would become my new graduate advisor upon Wu’s departure from UAH in 1988. Professor Anderson was essential to my emotional survival during several very difficult years. He passed away on Christmas Day, 1998.
- ↑ While this is how we characterized the reaction at the time, it is my understanding now that this description may not be rigorously correct.
- ↑ At sufficiently high temperatures, carbonates will typically decompose into a corresponding oxide plus carbon dioxide, the latter simply escaping into the air.
- ↑ Part II of this narrative will include some “analysis” of test results from the University of Houston. Data will be read using the same methods originally learned by Wu as a student there and later taught to me.
- ↑ Some of the original source code Media:020GDA3andTRUENP.pdf.
- ↑ UAH lab notebook, including cover and pages from December 1986 to January 1987 Media:030UAHuntsvilleLabNotebook.pdf.
- ↑ Processing conditions were most consistently recorded on the sample containers, few of which I was able to recover.
- ↑ Results of recovery of old data files Media:040OldFloppiesInFolders.zip.
- ↑ It should be noted here than any middle school level physical science book will, in its introduction to chemistry, emphasize the fact the elements in the same column (aka, group or family) of the periodic table, such as is the case with barium, strontium, and calcium, have very similar chemical properties. This should be kept in mind as the focus eventually turns to the first element in the formula – lanthanum.
- ↑ Often the transitions were broad or incomplete, in hindsight probably limited by incomplete reaction of the samples at the relatively low 999 °C temperature. The preferred processing temperatures for these materials at that time was closer to 1150 °C.
- ↑ See on page 255 of Media:060RLM1224MengArchiveWaggettMeeting.pdf a copy of the paper published in 1981 by Er-Rakho et al. describing La3Ba3Cu6O14 and related compositions with partial substitutions of La with various lanthanides and similar elements, including yttrium. L. Er-Rakho , C. Michel, J. Provost, B. Raveau. “The Oxides La3-xLnxBa3Cu(II)5-2yCu(III)1+2yO14+y.” Journal of Solid State Chemistry 37 (1981): 151-156.
- ↑ Indicative of the by then unremarkable La1.8Ba0.2CuO4 phase.
- ↑ N. Nguyen, F. Studer, B. Raveau. “Oxydes ternaires de cuivre a valence mixte de type K2NiF4 deficitaires en oxygene: Evolution progressive d’un etat semi-conducteur vers un etat semi-metallique des oxydes, La2-xSrCuO4-x/2+∂.” J. Phys. Chem. Solids 44.5 (1983): 389-400.
- ↑ As of this writing (December 2014), it has been a couple of years since I have seen Daniel. At our last meeting, he was hoping to soon retire from NASA Kennedy to a farm in Jackson County, Alabama.
- ↑ Until 1996, helium reserves in the United States were still controlled by the U. S. Department of Interior, from which we made our LHe purchases.
- ↑ My memory is fuzzy on this, but failure to place the order in time might have been an error on my part.
- ↑ As of this writing (December 2014), my last contact with Anthony “Tony” Xidis (January 2012) had him in Steubenville, Ohio by way of Kent State (graduate school); Minneapolis; Cleveland; Easten, PA; Bonaire, GA(?); and Florida.
- ↑ Ironically, having not yet seen a copy of the Bednorz-Müller paper, I was unaware that they had used coprecipitation. Daniel, Tony, and I had some horrific failures before we realized that oxalates were the most favorable approach.
- ↑ I should also mention here that there were a few likely relevant conversations with friend and chemistry student Raymond Cronise. While I have no recollections of the specific topics discussed, he perhaps might. As of this writing (December 2014), Ray still resides in Huntsville, Alabama.
- ↑ These “finely ground” samples continue to be the emphasis through the 13th of January Media:030UAHuntsvilleLabNotebook.pdf, pages 18-21.
- ↑ Samples were generally checked with a simple ohmmeter before preparing them for resistivity testing (cutting into a small bar, attaching four fine platinum leads with dots of indium, and loading into the “probe”). However, if the ohmmeter showed the sample to be insulating, no other testing was performed. Thus was the case here.
- ↑ See, for example, page 35 from Media:060RLM1224MengArchiveWaggettMeeting.pdf where Meng testifies in her 1993 declaration that “because the [Huntsville] test results were stated by Dr. Chu to be too poor to support a publication, he [Chu] directed me to prepare higher quality La-Sr-Cu-O samples for evaluation,… delay[ing] the time at which I could begin preparation of samples of Y-Ba-Cu-O which had been assigned to me.” Indeed, in our early work with La-Sr-Cu-O, our transitions did not complete until around 12 K, much like the original Bednorz-Müller results. While their results were adequate for a Nobel Prize, ours, in the assessment of Chu and Meng, are suggestive of incompetence (or at least a level of competence well below that of Meng). The reader is urged to closely examine the section devoted to Sample J-31 when seeking to discern Chu’s standards for “quality.”
- ↑ Another name from the Space Sciences Lab associated with Wu’s trips there was Alice Jones, but I cannot specifically connect her with any particular kind of work.
- ↑ UAH samples for Hall effect measurements Media:080HallSamples.jpg.
- ↑ Hall effect measurement results retrieved from the UAH lab Media:090HallEffect.pdf. Only pages showing dates have been included.
- ↑ In hindsight, it appears at least possible that Wu’s brief interest in substituting for the copper might have been the results of conversations with Chu. These samples happen to coincide with the 12 January 1987 filing of Chu’s first patent application on the copper oxides (copy to be linked later). That application includes substitutions for copper, in part or in whole, with “bismuth, titanium, tungsten, zirconium, tantalum, niobium, and vanadium or a mixture of one or more of these elements.” On the other hand, given that there is no overlap between these elements and the copper substitutions tested in Huntsville, I am inclined to suggest that this is evidence that there was correspondingly minuscule coordination between the two labs. More on Chu’s “carefully planned substitution program” later.
- ↑ See, for example, M. K. Wu, J. R. Ashburn, P. A. Curreri, and C.W. Chu. “High Pressure Study of Immiscible GaBi Alloys Solidified in Low Gravitational Field.” Proceedings of the Symposium on Material Processing in Reduced Gravity Environment (1986) and M. K. Wu, J. R. Ashburn, P. A. Curreri, and W. F. Kaukler. “Electric Properties of Al-In-Sn Alloys Directionally Solidified in High and Low Gravitational Fields.” Metallurgical Transaction A 18 (1987): 1511. The mercury sample might have been prompted by the concurrent interest within NASA’s Space Sciences Lab in II-VI semiconductors, especially HgCdTe. See, for example, a paper by two individuals mentioned elsewhere in this narrative: G. L. E. Perry, F. R. Szofran. “Highly Automated Optical Characterization with FTIR Spectroscopy.” NASA Technical Memorandum TM-100379 (September 1989) <http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19890020141.pdf>.
- ↑ Of course, my excuse would be that I had just completed my first Introduction to Solid State Physics class.
- ↑ I would refer the reader to basic textbooks on solid-state physics or crystal chemistry for discussions of atomic and ionic radii and their applications.
- ↑ More precise sources of ionic radii often include numbers as a function of crystal coordination, spin state, and so forth. Since I was not seeking that level of precision, I typically used the most commonly provided 6-coordination values.
- ↑ This was noted independently by Bednorz and Müller in an article published soon after. My preprint, dated 15 April 1987, is marked K. A. Müller, J. G. Bednorz. “The Discovery of a Class of High-Tc Superconductors.” Science 237 (1987): 1133.
- ↑ Linked copies of Chu’s various patent applications will be included later in this narrative.
- ↑ At first glance, the reader might question how “squeezing” a smaller atom into a given site in the crystal lattice previously occupied by a larger atom could reasonably simulate pressure. This is a valid question, but the answers concern matters not necessarily trivial (overlap of electronic orbitals, band structures, densities of states, and the like). I might defer to Chu, but so far I have yet to find any meaningful clarifications of what some of his superiors at Houston designated “the Chu Effect.” (see Carlos Byars, “Discovery May Earn Billions, Nobel for UH,” Houston Chronicle, 16 February 1987). Incidentally, given that there are currently about 100 known chemically or structurally unique copper oxide superconductors, it should be a relatively simple but tedious task to determine if there is indeed a correlation between critical temperatures and interatomic distances (presumably between the copper and oxygen). Surely someone stands to gain by actually verifying “the Chu Effect.” For some guidance on how this process might be conducted, the reader is referred to Appendix E.
- ↑ As reported by A. L. Robinson in Science 235 (1987): 531, Georg Bednorz and Karl Müller, Nobel Prize winners for their discovery of the first copper oxide high-temperature superconductor, correctly reference ionic radii. See K. A. Müller, J. G. Bednorz. “The Discovery of a Class of High-Tc Superconductors.” Science 237 (1987): 1133 for an example of this. Similarly, a report in Superconductivity News (March 1988, p. 16), in describing Allen Hermann’s discovery of the thallium-based copper oxide superconductor that established yet another high-Tc record, concluded with this (my emphasis): “Herman’s (sic.) thallium discoveries came from the dual-pronged benefit of a different processing step and the judicious use of periodic table information – valence states and ionic sizes. In all probability the next breakthrough will also require similar intelligence, foresight, and skill.” Ironically, earlier in the same paragraph, it erroneously describes YBCO as “true insightful discovery” prompted by “Chu’s pressure experiments.” It should be noted that Superconductivity News is no longer in publication and may be difficult to locate. It was the product of Superconductivity Publications, Inc. of Cranford, New Jersey, C. J. Russell Managing Editor, ISSN 0897-2427.
- ↑ Chu references atomic radii in his patent applications (to be covered in more detail shortly) on the following pages: 12 January 1987 (page marked 6), 27 January 1987 (page marked 9), 6 February 1987 (page marked 11), and 26 March 1987 (pages marked 13-14).
- ↑ The table from Kittel is referenced here as a matter of convenience because it just happens to be an example of a periodic table where lanthanum is depicted under yttrium. Because the lanthanides (the series from cerium to lutetium) actually fall between lanthanum and hafnium (to its right), many tables will show lanthanum as leading off the lanthanide row and lutetium in its place under yttrium and between barium and hafnium.
- ↑ J. R. Ashburn. Yttrium Barium Copper Oxide: The Formulation and Magnetic Properties of a 93 K Superconductor. Huntsville, AL: UAH (December 1990). Chapter 2 provides the technical description of the details leading up to the formulation of Y1.2Ba0.8CuO4 Media:110AshburnDissertationCh2.pdf.
- ↑ F. S. Galasso. Structure, Properties, and Preparation of Perovskite-type Compounds. Oxford: Pergamon Press, 1969. It was not until the time of this writing (December 2014) that I discovered that Galasso later (1990) penned a book entitled Perovskites and High Tc Superconductors.
- ↑ Wu, over the course of the first few weeks after the discovery but before our first “confrontation,” will, on at least a couple of occasions, tell the story that he prompted me to create this chart. However, my recollection of the chart in this book and its remarkable similarities with my hand-drawn version (to be shown shortly) would suggest that his memory was in error. Fortunately, after challenging him on this matter, an encounter to be covered in more detail later, he seemed to have corrected this aspect of his account, at least when within earshot of me.
- ↑ F. S. Galasso. Structure, Properties, and Preparation of Perovskite-type Compounds. Oxford: Pergamon Press, 1969, page 15.
- ↑ See Chapter 2 from J. R. Ashburn. Yttrium Barium Copper Oxide: The Formulation and Magnetic Properties of a 93 K Superconductor. Huntsville, AL: UAH (December 1990). Media:110AshburnDissertationCh2.pdf.
- ↑ One example of a packing condition averaging radius instead of volume that was overlooked at the time is Galasso’s tolerance factor (p.16). Galasso attributed the original to A W. Sleight, R. Ward. Inorg. Chem. 1 (1962): 790.
- ↑ A few examples are included here Media:140OptimalInterAtomicDistancesWu.pdf. This description of Wu’s will tend to fade after our March 1987 confrontation.
- ↑ Similarly obtuse descriptions by Wu appear in: M. K. Wu. “Study of Some Superconducting and Magnetic Materials on High Tc Oxide Superconductors.” Final Report, Grant No. NAG8-032 (October 1987); M. K. Wu, UAH Research Proposal No. 87-284 (May 1987), submitted to Dr. James Ionson, SDIO/T/IS, Pentagon; and, as communicated by Wu to C. Y. Huang – C. Y. Huang. “Discovery of High-Temperature Superconductors.” Proceedings: EPRI Workshop on High-Temperature Superconductivity 5.3-5.10 (1988).
- ↑ See Chapter 2 from J. R. Ashburn. Yttrium Barium Copper Oxide: The Formulation and Magnetic Properties of a 93 K Superconductor. Huntsville, AL: UAH (December 1990). Media:110AshburnDissertationCh2.pdf.
- ↑ On the 18th of January, a couple of other samples will be examined testing mixes of larger and smaller ions compensating for each other’s sizes, namely samples of La1.8(Ba0.5Ca0.5)0.2CuO4 and La1.8(Na0.6K0.4)0.2CuO4 (UAH lab notebook, page 23). These marked the first application of my weighted volume idea. Often the calculated results would be rounded to ratios of small integers (1:1 and 3:2 here). This pattern will be repeated with YBCO. Note also that no sample with Na absent of K or vice versa is ever made. Incidentally, La2-xKxCuO4 (x>=0.06) is later reported as superconducting by Keane et al. of Argonne National Labs. Preprint: P. M. Keane, D. G. Hinks, and J. D. Jorgensen. “Synthesis, Structure, and Superconductivity of La2-xKxCuO4.” submitted to J. Solid State Chem. <http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/28/017/28017409.pdf>. Similarly, Superconductivity News 1.9 (1988): 4 reported that the University of California San Diego and the DuPont Corporation “synthesized a new superconducting oxide based upon alkali [metal] doping for lanthanum in La2CuO4.” M. A. Subramian and coworkers reported similar results. M. A. Subramian, J. Gopalakrishnan, C. C. Torardi, T. R. Askew, R. B. Flippen, A. W. Sleight, preprint submitted to Science (1988).
- ↑ A survey of the literature quickly reveals that sub-oxides, C3O2 for example, are compounds whose difficult synthesis and inherently low stability would make them poor targets for anyone seeking to make a practical high-temperature superconductor.
- ↑ USPTO YBCO patent interference final decision after request for reconsideration Media:160FinalDecision1999.pdf.
- ↑ “Guy Allen Smith Obituary.” Huntsville Times (22-24 December 2014). <http://obits.al.com/obituaries/huntsville/obituary.aspx?pid=173561974>.
- ↑ My pocket calendar from January of 1987 Media:170Calendar1987January.pdf.
- ↑ As of my last contact with Jones Hamilton, he was still living in the Huntsville area and working in the McMorrow Labs (Building 5400) on Redstone Arsenal.
- ↑ I acknowledged him as such in my dissertation.
- ↑ Letter from Jones Hamilton describing his recollection of the events of January 1987 Media:180LetterJonesHamilton.pdf. While my memory of some of the details varies somewhat from Jones, the basic elements of his story are correct. His memory of the location of the calendar (given to me by Daniel Shultz, incidentally) is incorrect, however. It was located on the wall in the furnace room behind our main lab.
- ↑ W. J. Cook, R. Z. Chesnoff, M. Lord, P. Dworkin, J. Seamonds, M. Bosc. “Revolutionary Superconductors Made Through a New Alchemy: Seeking the Perfect Wire.” U.S. News & World Report 102.18 (11 May 1987): 66-71.
- ↑ There is some uncertainty on my part in attempting to distinguish Wu’s handwriting from Torng’s.
- ↑ Concerning his other memories, while Daniel did participate in the production of several of our samples during that period, he was not specifically involved with the very first yttrium samples. My recollection is that he did not witness the first tests on 29 January but was present during some of our tests soon after returning from Houston, including (as he notes) some of our first demonstrations of magnetic levitation.
- ↑ Fine et al. would later demonstrate superconductivity in a lanthanum-deficient phase with an optimal composition near (La0.95[ ]0.05)2CuO4. Thus, in hindsight, it seems likely that the mercury had completely evaporated. Fortunately, I did not know that at the time. M. Fine, M. Greenblatt, S. Simizu, S. A Friedberg. In Chemistry of High-Temperature Superconductors. Ed. D. L. Nelson, M. S. Whittingham, T. F. George. Washington: American Chemical Society (1987) 95.
- ↑ The analog furnace controller actually topped out at 999°C (three digit readout). The 996°C figure was based upon a later direct measurement of the point in the furnace where samples were usually positioned.
- ↑ I will later discover that Houston was one of them (to be covered in Part II of this narrative). I must also confess that my approach was not particularly disciplined, perhaps a reflection of my sometimes competitive nature.
- ↑ Wu informed me years ago that they ended up with his then friend C. Y. Huang. C. Y. has since indicated to me that he has no knowledge of the whereabouts of those original YBCO samples.
- ↑ Thus, I cannot speak to the specific information that was relayed, except that I seem to recall the (up to that time in our lab, little-used) word “yttrium,” as the pronunciation that came across as “eetrium” stuck in my mind. However, I must confess a bit of uncertainty whether or not my recollection was actually of a Wu phone call on that day or perhaps in the weeks to follow.
- ↑ Robert Hazen’s book, which will be covered in great detail later, mentions (page 48), “Chu and his collaborators complained about what appeared to be preferential treatment given to their New Jersey rivals [Bell Labs].”
- ↑ Robert Cava alluded to these fears when he described the start of 1987 this way: “Things were already getting almost out of control – there were stories circulating about spies and secret late-night telephone calls; we probably will never know exactly what was going on.” R. J. Cava, “Oxide Superconductors,” J. Am. Ceram. Soc. 83.1 (2000): 5-28.
- ↑ And possibly others, perhaps Professor Larry Smalley and Dean of the School of Science Harold Wilson. Dr. Smalley passed away on January 16, 2010. If my memory serves me, Dr. Wilson passed away in 1992.
- ↑ From the patent interference proceedings, Ruling Meng’s Exhibit H, excerpts from her lab notebook beginning late January 1987 Media:250MengExhibitH.pdf.
- ↑ Gina Kolata. “Yb or Not Yb? That is the Question.” Science, 236 (8 May 1987):663-4. <http://science.sciencemag.org/content/236/4802/663>.
- ↑ See page 23 in the UAH lab notebook Media:030UAHuntsvilleLabNotebook.pdf.
- ↑ See Media:150Y115Ba085CuO.jpg.
- ↑ R. Pool. “Superconductor Credits Bypass Alabama.” Science 5 (August 1988): 655-657. <http://science.sciencemag.org/content/241/4866/655>.
- ↑ See Media:220Y12Ba08CuO.jpg.
- ↑ Melissa Ford Thornton, “Dawn of Discovery: UAH Discovery Stuns the World,” UAH Magazine 1.1 (Winter 1988): 4-7.
- ↑ Upon obtaining Meng’s exhibits in the process of the patent interference, I knew immediately from the precision of the ratios that they were mine. However, it quite literally took years of on-and-off efforts for me to finally figure out the mapping of compositions, reproduce my errors, and recreate the compositions.
- ↑ I have more recently realized that I may have been leaning at the time towards the idea that the size of the lanthanum ion was the ideal.
- ↑ Purchased through my high school physics teacher, an inspirational lady by the name of Dottie Dale.
- ↑ A mix of the CRC value for lutetium and yttrium and the Kittel value for barium will round to the Meng result, but I cannot say with any certainty that such is what I did at the time.
- ↑ Stein, Mark A. “Column One: Trapped in His Own Shadow: Paul Chu.” Los Angeles Times Magazine (9 July 1993). <http://articles.latimes.com/1993-07-09/news/mn-11556_1_paul-chu>. Accessed 6 March 2015.
- ↑ Test results on what may be sample LYB-3 from Meng’s notes Media:305LYB3FromMengsNotes.jpg.
- ↑ See the page marked H52 from Media:250MengExhibitH.pdf.
- ↑ The reversed numbering is not the case with Meng’s notes.
- ↑ Somewhere there was an article or book referring to YBCO by this name, but I have not been able to locate it. Of course, since the YBCO discovery, the new “Holy Grail” is room temperature superconductivity.
- ↑ Why do I get the terrible sense that my sarcasm will eventually be quoted out of context?
- ↑ Frederick H. Katayama. “Wu? Chu? Or Who?” Fortune (13 February 1989). <http://archive.fortune.com/magazines/fortune/fortune_archive/1989/02/13/71633/index.htm>. Accessed 6 March 2015.
- ↑ The original YBCO paper Media:310OriginalYBCOPaper.pdf. M. K. Wu, J. R. Ashburn, C. J. Torng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang, C. W. Chu. “Superconductivity at 93 K in a New Mixed-Phase Y-Ba-Cu-O Compound System at Ambient Pressure.” Phys. Rev. Lett. 58 (1987): 908-910.
- ↑ Some elaboration on curious statements such as “pressure reduces the lattice parameter and enhances the Cu3+/Cu2+ ratio” might have been productive.
- ↑ Hor, in his deposition in the Hor-Chu case (see Appendix D), will also indicate that only in hindsight could Chu claim that he was seeking some phase other than the K2NiF4 structure (aka “214”).
- ↑ From A. Fisher. “Superconductor Frenzy.” Popular Science (July 1987): 54, 58, and 87, Chu is quoted as saying (my emphasis), “Finally, we decided to try a material with a new crystal structure: yttrium-barium-copper oxide” as if to suggest that not only was a new structure intended but also that it was somehow known or at least predictable prior to its fabrication and characterization. Fortunately, the story that a different crystal structure was intended was less emphasized with the passage of time.
- ↑ Consider the simple example of the reaction of two parts (as measured by the number of atoms) sodium and one part chlorine. Since table salt (NaCl) requires one part of each, excess sodium remains. Thus, such a reaction would yield a mixture of sodium metal and table salt, not “Na2Cl.” In the same way, when the oxides of Y, Ba, and Cu are mixed and reacted, they can yield (at equilibrium) up to three phases (ignoring variations in the oxygen). Among the now-known phases are the 90 K superconductor YBa2Cu3O7, the insulating “green phase” Y2BaCuO5, the insulating black compound BaCuO2, and any of the starting products Y2O3, CuO, or BaO (the latter after decomposition of the carbonate, in our case). The Y1.2Ba0.8CuO4 formula responsible for the 90 K discovery yields a mix consisting of roughly 75% by volume the insulating “green phase” Y2BaCuO5 and the remainder (black specks in the green matrix) mostly the 90 K superconducting phase YBa2Cu3O7 (often referred to as Y123 or simply 123).
- ↑ See Media:310OriginalYBCOPaper.pdf.
- ↑ Incongruent melting occurs when the initially solid material separates into a liquid and separate solid, each with elemental proportions different from the starting mix. The liquid can sometimes settle, thus further undoing the original mixing and yielding an even more inhomogeneous final product.
- ↑ See page 2 of Gleick, James. “In the Trenches of Science.” New York Times Magazine 136 (16 August 1987): 28. <http://www.nytimes.com/1987/08/16/magazine/in-the-trenches-of-science.html> Accessed 19 May 2015.
- ↑ Including Vince Sunderdick, if my memory serves me.
- ↑ The 12 January 1987 Houston patent application Media:320PatentApplication19870112.pdf.
- ↑ My aforementioned 60th edition of the CRC Handbook of Chemistry and Physics includes superconducting transition metal oxides for the following: Ti, W, Nb, V, Zr. Obviously, Cu was essential. Chu had substantial previous experience with Ba(Pb,Bi)O3 (see his papers from the mid-1990s to be covered later in this narrative). See also “Open discussion of papers by Chu and Muller.” Proc. Nati. Acad. Sci. USA 84 (July 1987): 4683-4688 (a discussion [from] the symposium “Interfaces and Thin Films,” organized by J. Armstrong, D. E. Eastman, and G. M. Whitesides, March 23 and 24, 1987, at the National Academy of Sciences, Washington, D. C.) for a chart showing many of these superconducting transition metal oxides along with discussions with Chu about Ba(Pb,Bi)O3.
- ↑ With the isolated exception of the relatively small class of electron-doped copper oxides, most notably Nd2-xCexCuO4.
- ↑ The importance of including the “mixtures” in this tally cannot be ignored. The highest critical temperature phases of the more recently discovered bismuth and thallium-based copper oxide superconductors both require “mixtures” of the “M” elements.
- ↑ R. Pool. “Superconductor Credits Bypass Alabama.” Science 5 (August 1988): 655-657. <http://science.sciencemag.org/content/241/4866/655>.
- ↑ In the original YBCO patent interference, Meng’s notebooks were cherry-picked to support the Houston account. However, in a subsequent related case beginning around 2006, more complete records became available Media:330MengMissingPagesPart2.pdf. To Meng’s credit here, I very recently noted that she had written down ionic radii for K, Cu, La, and Ba (page H488) sometime in the late November to early December 1986 timeframe. Below those four elements are separately listed Ba, Pb, and Bi, as in the oxide superconductor Ba(Pb,Bi)O3 (see previous footnote and recall Wu’s preference for “familiar” elements), a material not entirely unlike YBCO in its ugly heritage (per conversations with Houston graduate Dale Harrison, still living in Houston at the time of my last contact with him).
- ↑ A more complete collection obtained around 2009 of the pages from Meng’s notebook, part 1 Media:340MengMissingPagesPart1.pdf.
- ↑ And is further indication of just how largely nonexistent was any coordination between Houston and Huntsville. Simply working from the same reference materials in (La0.9Sr0.1)2CuO4 and (La0.9Ba0.1)2CuO4 and working up the columns of the periodic table, both groups will independently produce and test many identical or nearly identical compositions, in particular many of the same or similar failures. Beyond working from the same reference points and employing (albeit clumsily) the same basic chemistry, there is no indication that the parallels between samples made and tested by the two groups would be any more similar than any other randomly selected groups working the copper oxide materials at the time (except that some groups more savvy in crystal chemistry might have made generally more intelligent selections).
- ↑ It should be noted that test results on these insulating samples will not be found among the Houston charts. As I pointed out with the Y-Sr-Cu-O sample made on January 28th in Huntsville, it was customary to first do a quick resistance check with a simple ohmmeter on new samples. A very high resistance (insulating) meant that there was no need to prepare a sample for additional testing.
- ↑ For more on “the green phase,” see the chapter by the same name in Robert Hazen’s The Breakthrough, to be covered in Part II.
- ↑ Ruling Meng’s 1990 declaration Media:350MengDeclarationAndExhibitA1990.pdf.
- ↑ I have been told by some who have seen the original notebook page that the suspicious composition is written in a color of ink different from the surrounding notes; I have never personally seen anything but a photocopy. The topic is raised briefly in the Meng deposition (page 96, the archive of Meng’s testimony will be linked below).
- ↑ Ruling Meng’s 1993 declaration Media:360MengDeclaration1993.pdf.
- ↑ Incidentally, the previously linked document “Materials for Meeting with Gordon Waggett, May 9, 2007” Media:060RLM1224MengArchiveWaggettMeeting.pdf, a package of materials assembled in rebuttal of Meng’s perjury confession (i.e., for the purpose of establishing her initial testimony as true) includes both the redacted (submitted with her 4 December 1990 declaration) and unredacted versions (submitted with her 22 February 1993 declaration). See pages 13 and 57 of the pdf file.
- ↑ R. Pool. “Superconductor Credits Bypass Alabama.” Science 5 (August 1988): 655-657. <http://science.sciencemag.org/content/241/4866/655>.
- ↑ The curious reader might wonder if the date atop Meng’s 1993 Exhibit F was a reconstruction or if the original (or unmodified copy of the original) was still available at the time. For what it is worth, the “restored date” appears to nicely overlay the area of redaction in the 1990 Appendix A. Thus, my assumption is that the 15 January date is indeed original.
- ↑ 27 January 1987 University of Houston patent continuation Media:380PatentContinuation19870127.pdf.
- ↑ 6 February 1987 University of Houston patent continuation Media:390PatentContinuation19870206.pdf.
- ↑ Except that I had rejected it because its ionic radius is too close to copper. To my knowledge, there are no known copper oxides containing scandium as of this writing, certainly none of note.
- ↑ 26 March 1987 University of Houston patent continuation Media:410PatentContinuation19870326.pdf.
- ↑ By claiming substantial portions of the periodic table, Chu’s 26 March 1987 application covers or very nearly covers several important copper oxide superconductors discovered later by other groups, in particular those based upon mercury and bismuth. Even with the Texas Center for Superconductivity coming into existence, one can only speculate how many years (centuries? millennia? geologic eras?) might be required to search the space covered by the patent, especially when ratios and processing conditions are included. Incidentally, it should also be noted even Chu’s much more restrained 12 January 1987 application covers the original Bednorz-Müller composition for which they subsequently won the Nobel Prize. Also, the USPTO, in its infinite wisdom, will eventually award Chu US Patent No. 7,056,866 B1 in 2006.
- ↑ One can only wonder how it would not have been potentially more lucrative to file a patent for compound A, where A can be any mixture of C, H, O, N, S, P, and maybe another few selected elements, thus likely claiming future cures for most leading causes of sickness and death in the world today.
- ↑ The Emilio Segrè Visual Archives <http://www.aip.org/history-programs/niels-bohr-library/photos/aps-d1>. Photo description includes the statement “People in the photo are unidentified.” Incidentally, the person immediately to my right is C. J. Torng.
- ↑ While legal in Alabama, I confess that this was unethical. However, I was just 22 years old, embroiled in a situation that was spiraling out of control, and feeling forced to fight fire with fire. Recording of my 6 April 1987 confrontation with Wu Media:430WuConfrontationSpring1987.mp3.
- ↑ I am working from memory here, as I am personally not fond of listening to the track. The reader is encouraged to check me.
- ↑ Again, I am working from memory.
- ↑ Dr. Roy passed away in 2010.
- ↑ Dr. Bhalla is, as of this writing, at the University of Texas at San Antonio.
- ↑ At last check, Robert Pool is a freelance science writer in Florida.
- ↑ Years later, Roy informed me that he was so resentful of the evasive answers of his interviewees that he never made copies and specifically refused to have them listed as items for sale through the Materials Research Society. My notes indicate that a letter of Roy’s that appeared in Chemical and Engineering News may make reference to the tapes (Chem. Eng. News 66:38 (1988): 2-3).
- ↑ See Media:170Calendar1987January.pdf.
- ↑ Chu’s desk calendar Media:440ChusCalendarFromRLM1224.pdf.
- ↑ Understandably a priority in his current position over the Texas Center for Superconductivity but not so much when his lab consisted of no more than a dozen people.
- ↑ Excluding, of course, combinations where traces of elements that only degrade the superconductivity or, at a minimum, contribute nothing to the superconducting properties could be included to count them among the superconductors.
- ↑ Having already noted the La3Ba3Cu6O14 Houston-made sample tested in Huntsville in mid-December whose formula is traceable to the aforementioned Er-Rakho et al. paper retrieved by Wu in early December describing partial substitutions of lanthanum for yttrium in a parent material of La-Ba-Cu-O, I an inclined to speculate that this is related to why Chu felt it necessary to specify complete replacement. Without the surrounding context of a potential partial replacement, the word “replacement” alone would have generally been interpreted as complete replacement. However, Chu seems to be putting some separation between his “idea” and the Er-Rakho work, even as he declines to draw attention to it. This all creates a certain degree of irony when we consider that Robert Hazen’s discussions of Chu as a potential co-recipient of the 1987 Nobel Prize would largely reduce Chu’s contributions to a simple extension of partial replacement to complete replacement, all to ultimately create a crystal structure no one could have reasonably anticipated (the “2-1-4” ratios of the starting materials certainly indicate that the eventual structure, not “2-1-4,” was unanticipated). I would also note that it is effectively partial substitutions of certain alkali earths for lanthanum in a parent material of La2CuO4 that trigger superconductivity in those compounds (and only partial substitutions, neglecting the not-easily-made superconductor Sr2CuO3 discovered with great pains years later). Thus, Chu’s claim to have somehow known in advance that only complete substitutions of yttrium for lanthanum would be most favorable for superconductivity at higher temperatures is difficult to believe.
- ↑ Anthony Ramirez. “Superconductors Get Into Business.” Fortune (22 June 1987).
- ↑ T. H. Maugh. “New Material Further Increases Superconductor Temperature.” Los Angeles Times (23 June 1987).
- ↑ Jon Van, “Hot New Finding On Superconductivity May Save Cool Cash.” Chicago Tribune (23 May 1987).
- ↑ The many national articles were apparently prompted by a 23 May 1987 Associated Press report entitled “Researcher Says New Materials Raise Temperature Level Needed for Superconductivity.”
- ↑ C. W. Chu. “Superconductivity above 90 K.” Proc. Nati. Acad. Sci. USA 84 (July 1987): 4681-4682, paper presented at the symposium “Interfaces and Thin Films,” organized by J. Armstrong, D. E. Eastman, and G. M. Whitesides, March 23 and 24, 1987, at the National Academy of Sciences, Washington, D. C. <http://www.pnas.org/content/84/14/4681.short>. Incidentally, this paper is among Chu’s earlier accounts establishing the 29 January discovery date to be subsequently abandoned by Houston in the patent interference.
- ↑ For more on “November 25, 1986,” see Appendix A.
- ↑ As of this writing, a scholar.google.com search for YBa2Cu3O7 yields 268,000 results.
- ↑ R. J. Cava, J. J. Krajewski, W. F. Peck Jr., B. Batlogg, L. W. Rupp Jr., R. M. Fleming, A. C. W. P. James, P. Marsh. Nature 336 (1988): 660.
- ↑ P. Marsh, R. M. Fleming, M. L. Mandich, A. M. Desantolo, J. Kwo, M. Hong, and L. J. Martinez-Miranda. “Crystal structure of the 80 K superconductor YBa2Cu4O8.” Nature 334 (14 July 1988): 141-143.
- ↑ P. Bordet, C. Chaillout, J. Chenavas, J. L. Hodeau, M. Marezio, J. Karpinski, E. Kaldis. “Structure determination of the new high-temperature superconductor Y2Ba4Cu7O(14+x).” Nature 334.6183 (1988): 596-598.
- ↑ M. Kato, M. Nakanishi, T. Miyano, T. Shimizi, M. Kakihana, K. Kosuge, J. Solid State Chem. 139 (1998): 266.
- ↑ A. O. Ayaş, A. Ekicibil, S. K. Çetin, A. Coşkun, A. O. Er, Y. Ufuktepe, T. Fırat, K. Kıymaç. “The Structural, Superconducting and Transport Properties of the Compounds Y3Ba5Cu8O18 and Y3Ba5Ca2Cu8O18.” Journal of Superconductivity and Novel Magnetism 24.8 (November 2011): 2243-2252. Some sources have reported critical temperatures around 100 K.
- ↑ T. Kruaehong, S. Sujinnapram, T. Nilkamjon, S. Ratreng, P. Udomsamuthirun. “Investigate the Properties of Y211 Doping Effect in the New Superconducting Y7Ba11Cu18Oy Compound.” Advanced Materials Research 770 (September 2013): 26-29.
- ↑ G. Duthie. Letters. The Scientist 2.13 (July 11, 1988): 14.
- ↑ See <http://www.tcsuh.com/people/facultypl/chu_paul/> and <http://www.tcsuh.com/pdf/cv/chu_paul.pdf>. Accessed 20 December 2014.
- ↑ R. Pool. “Superconductor Credits Bypass Alabama.” Science 5 (August 1988): 655-657. <http://science.sciencemag.org/content/241/4866/655>.
- ↑ Therapeutic despite the fact that Dr. Anderson, even with his “mature” age, defeated me routinely.
- ↑ For completeness, I am linking here all of the “pre-29 January” Meng lab exhibits from the original YBCO patent interference that I have not referenced elsewhere in this manuscript: Media:460MengExhibitB.pdf, Media:461MengExhibitC.pdf, Media:462MengExhibitD.pdf, and Media:463MengExhibitE.pdf. I have only a few digital versions of the “post-29 January” exhibits (they seemed of little relevance at the time I performed these scans and my constitution was waning). At least one of the post-discovery exhibits (Exhibit I) will be included at the appropriate point later in this narrative.
- ↑ My analysis of Meng’s testimony as submitted (with some minor more recent edits) to the UAH patent attorney in 1994 Media:470MengAccountAshburnAnalysis.pdf.
- ↑ Prompted when Hor and Meng find that they were never included as co-inventors on the Houston patent applications.
- ↑ While it was very much a bittersweet occasion, thanks are still due Drs. Carroll Johnson and Larry Smalley for initiating this recognition. It felt good to see my alma mater give their very best effort to make things right. As of this writing (2014), Dr. Johnson is still on the faculty of UAH. Dr. Smalley passed away on January 16, 2010.
- ↑ While the UAH team had not yet found Chu’s “later papers” from the mid-’90s, we did have Chu’s Novel Superconductivity paper (to be discussed in greater detail in Part II) that established the date of the discovery as 29 January 1987, contrary to the 1 February discovery story Houston presents in their side of the case. As communicated to me by Kelber, that paper was introduced into the final hearing (over multiple objections by the Houston attorneys asserting the long-since closure of the discovery period). All objections were overruled, and it seemed that a key leg of the Houston account had been destroyed. However, because that paper was not in the written record and there was no transcript taken, the two “replacement judges” could not benefit by it.
- ↑ Monetarily. For me, it represented vindication, at least until I came to appreciate that the patent interference process, as described by UAH attorney John Cates, is a “war of attrition.”
- ↑ Such words were quite out of character for me. However, it was equally rare to be asked to sell my integrity.
- ↑ I do not recall his precise choice of words.
- ↑ Slow cooling is also favorable to the formation of the 90 K YBCO superconductor.
- ↑ D. R. Clarke. “The Development of High-Tc Ceramic Superconductors: An Introduction.” Advanced Ceramic Materials 2(3B) Special Supplementary Issue (July 1987): 273ff, an American Ceramic Society Publication.
- ↑ R. S. Roth, K. L. Davis, J. R. Dennis. “Phase Equilibria and Crystal Chemistry in the System Ba-Y-Cu-O.” Advanced Ceramic Materials 2(3B) Special Supplementary Issue (July 1987): 273ff, an American Ceramic Society Publication Media:500ObviousYttriumAdvancedCeramicMaterials.jpg.
- ↑ A. Khurana. “Superconductivity Seen Above the Boiling Point of Liquid Nitrogen.” Physics Today 40:4 (1987): 17-23 Media:510ObviousYttriumPhysicsToday.jpg.
- ↑ Robert Hazen. The Breakthrough: The Race for the Superconductor. New York: Summit, 1988. This passage should be kept in mind as I later examine Houston’s very short-lived mid-January failed attempts with yttrium using a low barium concentration guaranteed to produce an insulating “green stuff” and a processing temperature unfavorably above the incongruent melting point of the YBCO superconducting phase.
- ↑ In the “Amended Memorandum and Order Entering Findings of Fact and Conclusions of Law” from Civil Action No. 4:08-cv-3584, Pei-Herng Hor vs. Ching-Wu “Paul” Chu, the court observed in their conclusions (page 38, my emphasis), “Dr. Hor bases his inventorship on his suggestion to Dr. Wu in late December 1986 or early January 1987 that Y be substituted for La in LBCO 214, but he has failed to offer enough corroborating evidence, especially considering that he relies in large part on Ms. Meng, who is in interested party and has even, at times, hinted that she herself conceived of that idea.” I would, at this point, urge the reader to reexamine his own personal history, his actions and whereabouts on or about January 1987, and consider the possibility that he, too, may have been a coinventor of the YBCO superconductor. This document and the rest of the Hor-Chu case will be examined further in Appendix D.
- ↑ C. W. Chu, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Huang, J. Bechtold. “Discovery and Physics of Superconductivity Above 90 K.” Novel Superconductivity: Proceedings of the International Symposium on Novel Mechanisms of Superconductivity. Ed. S. A. Wolf, V. Z. Kresin. New York: Plenum Press, 1987. 581-598. <http://link.springer.com/chapter/10.1007/978-1-4613-1937-5_68>.
- ↑ At equivalent oxidation states.
- ↑ My apologies for “x” mapping to the y-axis. These charts were actually created before I realized that my coordinates mapped to numbers in Chu’s early patent applications.
- ↑ Benign from a testing and discovery standpoint. Had the composition been overrich in barium and/or copper, low melting point phases would form, capable of coating superconductor grains and reducing the prominence of the measured resistivity drop.
- ↑ Even high-energy atomic oxygen has been explored by some for processing the copper-oxide superconductors.
- ↑ C. W. Chu, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Huang, J. Bechtold. “Discovery and Physics of Superconductivity Above 90 K.” Novel Superconductivity: Proceedings of the International Symposium on Novel Mechanisms of Superconductivity. Ed. S. A. Wolf, V. Z. Kresin. New York: Plenum Press, 1987. 581-598. <http://link.springer.com/chapter/10.1007/978-1-4613-1937-5_68>.
- ↑ To be precise, the Houston tests appear to be zero-field cooled (ZFC) tests, meaning that the samples were cooled to 4 K, a magnetic field was only then applied, and then the measurements were taken as the sample was warmed. This can be determined by the fact that the numbered curves in the raw data begin with the lowest temperatures. However, to avoid having to translate Chu’s references to transitions “beginning/starting” at certain temperatures, meaning the onset of superconductivity as a sample might be cooled, my narrative will proceed as if these were field-cooled (FC) tests. The validity of the discussion is unaffected by the simplification.
- ↑ C. W. Chu. “Superconductivity Above 90 K and Beyond.” Proceedings of the 10th Anniversary HTS Workshop on Physics, Materials and Applications. Ed. B. Batlogg, C. W. Chu, W. K. Chu, D. U. Gubser, K. A. Müller. Singapore: World Scientific, 1996. 17 Media:560ChuSCAbove90K.pdf.
- ↑ C. W. Chu. “High Temperature Superconductivity.” History of Original Ideas and Basic Discoveries in Particle Physics. Ed. H. B. Newman, T. Ypsilantis. New York: Plenum, 1996. 793. <http://www.springer.com/us/book/9780306452178>.
- ↑ C. W. Chu. “The Brief History of RBCO.” The 20th Anniversary of the Discovery of RBCO. Denver, 5 March 2007 <http://www.w2agz.com/Presentations/2007/03-05%20APS%20March%20Denver/> Media:600Chu-070305HTS-APSDenver.pdf.
- ↑ Paul C. W. Chu. “From BCS through HTS to RTS.” BCS@50. Champaign Urbana, IL, 12 October 2007 <http://www.conferences.uiuc.edu/bcs50/PDF/Chu.pdf> Media:610Chu-101207BCSat50-Urbana.pdf.
- ↑ Paul C. W. Chu. “HTS 20 Years later: Achievements, Promises, Challenges plus the New Fe-Based HTS System.” 2008 American Physical Society, April Meeting, St. Louis MO, 14 April 2008 <http://apps3.aps.org/aps/meetings/april08/Q1.00003.pdf> Media:620PresentationQ100003.pdf.
- ↑ C. W. Chu, L. Z. Deng, B. Lv. “Hole-Doped Cuprate High Temperature Superconductors.” Submitted on 16 Feb 2015. <http://arxiv.org/abs/1502.04686>. Accessed 14 March 2015. Accepted for publication in Physica C, Special Issue on Superconducting Materials. See text on page 6 and Figure 7 on page 45.
- ↑ M. K. Wu, J. R. Ashburn, C. J. Torng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang, C. W. Chu. “Superconductivity at 93 K in a New Mixed-Phase Y-Ba-Cu-O Compound System at Ambient Pressure.” Phys. Rev. Lett. 58 (1987): 908-910 Media:310OriginalYBCOPaper.pdf.
- ↑ Request from Houston the page stamped H806 (“Wu’s #1 LR-400 1-30-87”). “LR-400” is in reference to the Model LR-400 Lake Shore Cryotronics AC Resistance Bridge. Many such devices are also perfectly suitable for AC susceptibility measurements.
- ↑ As assortment of Houston test results collected by Ruling Meng Media:660RLM0572HoustonTests.pdf.
- ↑ But appeared to be in the transition to automated testing as evidenced by several printouts of automated data collection results among their documents and occasional notes of data file names on their charts.
- ↑ The origin on the charts is the center of the grid, often marked by hand with an “x.”
- ↑ The reader will simply have to contact me if verification is required on this one.
- ↑ The discontinuity in the original background data around 115 K appears to have been an offset adjustment of 0.1 mV that was not noted. This is apparent from the bouncing of the plotter pen and the fact that the curve perfectly resumes its previous slope.
- ↑ In most of the Houston magnetic susceptibility rests (both sample and background tests), a diamagnetic signal associated with a lead reference is visible around 5 to 7 K. The measured temperature may vary a degree or two because the very lowest temperatures are not accurately measured with a thermocouple (due to a flattening voltage curve). Houston’s lead reference, for whatever reason, appears to consistently generate a primary signal around 6 K and a second smaller signal about a half-degree lower. The curious double-dip fingerprint – visible only in the raw data, not in the more granular processed graphs – is helpful in its unambiguous identification.
- ↑ Media:650OriginalYBCOPaperMagSusc.jpg.
- ↑ Email from Peiherng Hor to Jim Ashburn dated 2 January 2012 Media:720HorEmail.jpg.
- ↑ Media:330MengMissingPagesPart2.pdf.
- ↑ T. Wada, N. Suzuki , T. Maeda, A. Maeda, S. Uchida, K. Uchinokura, S. Tanaka. “High Transition Temperature Superconductor LaBa2Cu3O7-y with Zero Resistance at 92 K.” Applied Physics Letters 52.23 (June 1988):1989-1991.
- ↑ Not to mention, an unsecured ferromagnetic particle may move about within the apparatus, driven by the applied field. Depending upon its location relative to the secondary “pickup” coil, the induced signal can even appear to be diamagnetic.
- ↑ Both effects are nicely captured in the Langevin paramagnetic equation which, with a “bias” due to hysteresis, does a decent job modeling the J-31 background signal.
- ↑ T. Wada, N. Suzuki , T. Maeda, A. Maeda, S. Uchida, K. Uchinokura, S. Tanaka. “High Transition Temperature Superconductor LaBa2Cu3O7-y with Zero Resistance at 92 K.” Applied Physics Letters 52.23 (June 1988):1989-1991.
- ↑ Chinping Chen, Lin He, Lin Lai, Hua Zhang, Jing Lu, Lin Guo, Yadong Li. “Magnetic Properties of Undoped Cu2O Fine Powders with Magnetic Impurities and/or Cation Vacancies.” Journal of Physics: Condensed Matter 21.14 (2009).
- ↑ Shu Li and Martha Greenblatt. “Chemistry and High Tc Superconductivity in the La-Ba-Cu-O System.” Technical Report No. 32, 1 Jul. 1987 - 15 Jul. 1988 (submitted to the Office of Naval Research), Rutgers University Department of Chemistry, New Brunswick, NJ.
- ↑ For reference, a perfect superconductor has a magnetic susceptibility Χm(SI) of -1. Strong ferromagnets can approach +106.
- ↑ It might also be notable that the furnaces within which these samples are processed often consist of coiled heating elements whose magnetic fields could have increased or otherwise altered the initial net magnetism within the sample.
- ↑ Jeff Hecht. “Science: High-temperature Superconductors are Unstable.” New Scientist 1871, (1 May 1993).
- ↑ Thin films are potentially an exception to this rule as the film is more susceptible over its volume to the effects of thermal expansion mismatch with the thicker substrate.
- ↑ While on the topic (see previous footnote), this effect is potentially the result of (or at least compounded by) thermal expansion mismatches between sample and contact.
- ↑ Sarcasm. Robert Cava’s “Oxide Superconductors” has more to say about the “Journal of Irreproducible Materials Science” and related topics. R. J. Cava, “Oxide Superconductors,” J. Am. Ceram. Soc. 83.1 (2000): 5-28.
- ↑ C. W. Chu. “The Brief History of RBCO.” The 20th Anniversary of the Discovery of RBCO. Denver, 5 March 2007 <http://www.w2agz.com/Presentations/2007/03-05%20APS%20March%20Denver/> Media:600Chu-070305HTS-APSDenver.pdf.
- ↑ I would note that thermal expansion mismatches between the component minerals of granite would not inherently lead to changes in the electrical and magnetic properties of those minerals as a simple consequence of cycling to low temperatures.
- ↑ I do not know if or when it will appear in the electronic records (see Appendix D for the links) given that it may not have been submitted in support of a motion, etc.
- ↑ Carlos Byars. “Discovery May Earn Billions, Nobel for UH.” Houston Chronicle, 16 February 1987.
- ↑ From the original YBCO Physical Review Letters paper, “The compounds investigated were prepared… through solid-state reaction of appropriate amounts of Y2O3, BaCO3, and CuO.” M. K. Wu, J. R. Ashburn, C. J. Torng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang, C. W. Chu. “Superconductivity at 93 K in a New Mixed-Phase Y-Ba-Cu-O Compound System at Ambient Pressure.” Phys. Rev. Lett. 58 (1987): 908-910 Media:310OriginalYBCOPaper.pdf.
- ↑ Robert Hazen’s book on the discovery (to be covered shortly) has a section devoted to this topic.
- ↑ D. H. Bowen. in High Pressure Physics and Chemistry I. ed. R. S. Bradley, Academic Press, Inc., New York (1963): 355-373.
- ↑ The reader is urged not to speculate that high-temperature superconductors have enabled time travel.
- ↑ All my ball-milling and coprecipitation work only to discover later that my actual objective was dirty mixed phase samples. What a waste of effort.
- ↑ Robert Hazen. The Breakthrough: The Race for the Superconductor. New York: Summit, 1988.
- ↑ Shorthand for the pure-phase YBa2Cu3O7 material.
- ↑ See Media:330MengMissingPagesPart2.pdf.
- ↑ Incidentally, Chu was, at the time (1986-1987), serving as director of Solid State Physics Program within the National Science Foundation, leading him to spend much time in Washington. From his writings, we know of at least two trips in January alone. From what I have been told (hearsay, I confess), Chu’s many commitments made it difficult for him to direct the day-to-day activities even in Houston, much less Huntsville, which would further explain why he had pathetically little knowledge of what we were actually doing in Alabama. It is perhaps notable that, at the time of the discovery, the UAH work was at least partially funded by NSF grants.
- ↑ M. D. Lemonick. “Superconductors! The Startling Breakthrough That Could Change Our World.” Time 129.19 (11 May 1987): 64-75. I have no idea what “chemical treatments” are.
- ↑ Well, that and Wu’s Y1.8Sr0.2CuO4 sample, to be precise.
- ↑ See Media:740MengExhibitG.jpg. As previously noted, my very specific recollection is that Exhibit G was penned after the arrival of Wu and myself in Houston on the 30th of January, not the 29th. Obviously the year was in error as well. More will be said about Exhibit G soon.
- ↑ I spoke with Robert Hazen by phone some time around 1989. He was very kind and attentive but confessed that an updated version of his book was unlikely since works of this nature necessarily capitalize on a timely release.
- ↑ Linda Garmon. “Race for the Superconductor.” NOVA, Boston: PBS WGBH, 29 March 1988 . For more on Chu’s dreams, see Gleick, James. “In the Trenches of Science.” New York Times Magazine 136 (16 August 1987): 28. <http://www.nytimes.com/1987/08/16/magazine/in-the-trenches-of-science.html?pagewanted=1> Accessed 19 May 2015.
- ↑ R. Pool. “Superconductor Credits Bypass Alabama.” Science 5 (August 1988): 655-657. <http://science.sciencemag.org/content/241/4866/655>.
- ↑ Media:480UAHSampleTestedInHouston19870130.jpg.
- ↑ Media:470MengAccountAshburnAnalysis.pdf.
- ↑ Meng’s 2006 “perjury” affidavit Media:760MengAffidavit2006.pdf.
- ↑ Hor’s 2006 affidavit Media:770HorAffidavit2006.pdf.
- ↑ Media:330MengMissingPagesPart2.pdf.
- ↑ Very late in the production of this manuscript, I realized that I could not find in any of the Houston documentation I possess a composition explicitly noted as La3Ba3Cu6O14, the composition from the Er-Rakho et al. paper that we associated with at least one of the Houston samples sent to Huntsville for testing in December 1986 (see Part I). For reasons unknown, J-36, whose metallic ratios are identical to La3Ba3Cu6O14 but was apparently not fabricated until early January 1987, is the closest match. It is curious that the Houston sample bottles I possess (at least one of which I presume to correspond to the test we identified as “La3Ba3Cu6O14 from Houston”) marked J-17, J-22, and either J-8 or J-18 (the latter label being too faded and soiled to clearly discern) can now be identified from the Houston notes as (La0.925Ba0.175)2CuO4 (J-17 and J-22) and either (La0.8Ba0.2)2CuO4 (J-8) or (La0.9Ba0.1)2CuO4 (J-18), compositions consistent with the 30 K transition we observed in our testing. It is also unclear to me why samples were ever sent to Huntsville in the first place (as we had no additional testing capabilities) unless Houston was looking for confirmation of something they believe to have observed. If a presumed indication of superconductivity at an unusually high temperature, then perhaps the composition they reported to us (La3Ba3Cu6O14) was not the true composition (recall the Yb story as well as Chu’s mid-’90s accounts that seem to have found it unremarkable that Wu would withhold the composition corresponding to our 29 January tests) but was simply one taken randomly from one of the papers in their possession (thus giving it some credibility). As of this writing, I have not located an entry in the Houston notes a sample having the La3Ba3Cu6O14 formula, but I must confess being a bit too weary to perform a very exhaustive search. See Media:050HoustonSamples.jpg, Media:330MengMissingPagesPart2.pdf, and page 225 of Media:060RLM1224MengArchiveWaggettMeeting.pdf.
- ↑ C. W. Chu. “High Temperature Superconductivity.” History of Original Ideas and Basic Discoveries in Particle Physics. Ed. H. B. Newman, T. Ypsilantis. New York: Plenum, 1996. 793. <http://www.springer.com/us/book/9780306452178>.
- ↑ For another example where Chu has conveniently associated the flawed J-31 magnetic susceptibility results with the J-36 x-ray diffraction, see C. W. Chu. “The Brief History of RBCO.” The 20th Anniversary of the Discovery of RBCO. Denver, 5 March 2007 <http://www.w2agz.com/Presentations/2007/03-05%20APS%20March%20Denver/> Media:600Chu-070305HTS-APSDenver.pdf. The J-36 identifier is clearly visible at the top of the XRD pattern.
- ↑ Shu Li and Martha Greenblatt. “Chemistry and High Tc Superconductivity in the La-Ba-Cu-O System.” Technical Report No. 32, 1 Jul. 1987 - 15 Jul. 1988 (submitted to the Office of Naval Research), Rutgers University Department of Chemistry, New Brunswick, NJ.
- ↑ My recollection is that because of the difficulties associated with 1) distinguishing the contributions of lanthanum and barium in the crystal lattice on the X-ray diffraction pattern and 2) identifying oxygen vacancies and their patterns, the much more involved neutron diffraction techniques are often preferred for precise determinations of these crystal structures. An example of this analysis can be found in W. I. F. David, W. T. A. Harrison, R. M. Ibberson, M. T. Weller, J. R. Grasmeder, and P. Lanchester. “The Structure of the Non-superconducting Phase La3Ba3Cu6O14+x and its Relation to the High-Tc Superconductor YBa2Cu3O7-δ.” Nature 328 (23 July 1987): 328-329.
- ↑ M. H. Chandehari and S. G. Brass. “Consecutive Inert and Oxygen Atmosphere Sintering in the Synthesis of LaBa2Cu3Oy with T(R = 0) Greater Than 90 K.” Journal of Materials Research 4 (Sep-Oct 1987):1111-1115.
- ↑ An initial processing in vacuum does have some utility in accelerating the decomposition of carbonates when among the raw materials.
- ↑ For reasons I cannot explain, Meng assigns the initial J-31 results to 11 January instead of 12 January. There were indeed earlier J-31 tests but on 7 January, not 11 January.
- ↑ See Media:330MengMissingPagesPart2.pdf.
- ↑ Media:790MengDeposition93.pdf. I should note that I did not knowingly or actively contribute questions for the Meng deposition. Thus, I can offer no explanation why so much of the examination was devoted to seemingly irrelevant questions on “calcium samples.” I can only speculate that the purpose was either 1) to ultimately refute Chu’s pressure gibberish supposedly connecting higher critical temperatures with reduced “interatomic distances” or 2) to keep Meng off balance (I am told that is why attorneys will ask their questions in seemingly random order).
- ↑ Media:470MengAccountAshburnAnalysis.pdf.
- ↑ Media:330MengMissingPagesPart2.pdf.
- ↑ Without frequent all-nighters, the later 26 March application would have required a bit longer to cover at that pace – about 500,000 years – assuming only one set of ratios and one set of processing conditions for each combination of elements.
- ↑ Recall YB-1 from mid-January where the right combination of elements but with unfavorable ratios and processing still yielded an insulating failure.
- ↑ See Media:480UAHSampleTestedInHouston19870130.jpg, for example, noting “Wu’s #2” in the top right.
- ↑ The reader should read Part I for a painfully detailed description of the “principles” and “rules” as they were worked in Huntsville.
- ↑ Sources report up to four (-2, +1, +2, and +4), but negative valences would never occur in an oxide, and +4 has only been reported in fluorides of mercury, thus leaving only two possibilities. My guess is that Meng is thinking of bismuth.
- ↑ See Media:480UAHSampleTestedInHouston19870130.jpg.
- ↑ See Media:440ChusCalendarFromRLM1224.pdf.
- ↑ C. W. Chu. “High Temperature Superconductivity.” History of Original Ideas and Basic Discoveries in Particle Physics. Ed. H. B. Newman, T. Ypsilantis. New York: Plenum, 1996. 793. <http://www.springer.com/us/book/9780306452178>.
- ↑ C. W. Chu. “Superconductivity Above 90 K and Beyond.” Proceedings of the 10th Anniversary HTS Workshop on Physics, Materials and Applications. Ed. B. Batlogg, C. W. Chu, W. K. Chu, D. U. Gubser, K. A. Müller. Singapore: World Scientific, 1996. 17 Media:560ChuSCAbove90K.pdf. I think the reader will find Chu’s description of the “stillborn LBCO-paper” (page marked 22) especially nauseating given the back-story of sample J-31.
- ↑ C. W. Chu. “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc.” IEEE Transactions on Applied Superconductivity 7.2 (1997): 80-89. <http://ieeexplore.ieee.org/xpl/login.jsp?tp=&arnumber=614424>.
- ↑ R. Pool. “Superconductor Credits Bypass Alabama.” Science 5 (August 1988): 655-657. <http://science.sciencemag.org/content/241/4866/655>.
- ↑ See pages H17 and H47 in Media:340MengMissingPagesPart1.pdf.
- ↑ One could dispute this under the assumptions that 1) yttrium was indeed discussed in early January (certainly possible), 2) Wu did indeed make the statement about doing what was “discussed previously” (also possible) and 3) his statement referred only to using yttrium. Of course, having rather firmly established that yttrium alone was far short of the necessary and sufficient information to achieve the discovery, Chu’s anecdote is inconsequential and Wu’s supposed use of the word “just” misses the mark, especially as we continue to examine Houston’s January yttrium failure(s) presumably patterned after “just… what we discussed previously.”
- ↑ C. W. Chu. “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc.” IEEE Transactions on Applied Superconductivity 7.2 (1997): 80-89. <http://ieeexplore.ieee.org/xpl/login.jsp?tp=&arnumber=614424>.
- ↑ I am not necessarily suggesting that the “mistake” is unintentional.
- ↑ See Media:740MengExhibitG.jpg and Media:250MengExhibitH.pdf.
- ↑ As mentioned earlier, it is highly implausible that the activities associated with Exhibit H started on 29 January such that Meng left space between “29” and “January” in order to add “-30” when those activities stretched into a second day. Otherwise, she would have had to routinely leave such space on every page of her notes. Furthermore, I have also demonstrated in Part I in great detail how I was the one who derived the unusual compositions that appear on the first three pages of Exhibit H, and my arrival with Wu, by all accounts, was on 30 January.
- ↑ Note that Meng’s numbering (beginning RLM1282) places this section (Media:250MengExhibitH.pdf) immediately following Media:740MengExhibitG.jpg (RLM1281).
- ↑ Compare Exhibit A from Meng’s 1990 declaration Media:350MengDeclarationAndExhibitA1990.pdf (the obfuscation of the date is actually acknowledged in the declaration) to Exhibit F Media:370MengExhibitF.jpg, which was included as an attachment to Meng’s 1993 declaration Media:360MengDeclaration1993.pdf.
- ↑ See page marked H476 from Media:330MengMissingPagesPart2.pdf.
- ↑ Before introducing the ytterbium “typo,” it was necessary to establish that ytterbium would not work. Given its similarities with yttrium, that was certainly a possibility. Only much later was it learned that ytterbium is indeed a suitable substitute for yttrium in the 90 K superconductors, although I understand that its synthesis is a bit more finicky.
- ↑ While some accounts examined in this document claim that Wu was shown specific compositions during the supposed New Year’s discussions, the fact that Houston subsequently failed with yttrium (twice by Chu’s accounting) before the Huntsville discovery indicates that the key – the reason UAH was successful while so many others including Houston, Bellcore, and Tokyo were not – is simply never addressed in the Houston stories.
- ↑ See page marked H476 from Media:330MengMissingPagesPart2.pdf.
- ↑ See Media:250MengExhibitH.pdf.
- ↑ See page marked H476 from Media:330MengMissingPagesPart2.pdf.
- ↑ See Media:250MengExhibitH.pdf.
- ↑ See Media:330MengMissingPagesPart2.pdf.
- ↑ Chu’s patent applications from January of 1987, to be covered later, appear to indicate a minimal processing time of at least 18 hours spanning multiple heating cycles.
- ↑ Contrary to Chu’s account emphasizing yttrium and ytterbium, priority was clearly given to yttrium and lutetium. Testing of ytterbium samples only became imperative to verify (or at least attempt to verify) that ytterbium would not work prior to doctoring the composition in the original submission of the YBCO paper. See Gina Kolata. “Yb or Not Yb? That is the Question.” Science, 236 (8 May 1987):663-4. <http://science.sciencemag.org/content/236/4802/663>.
- ↑ See Media:360MengDeclaration1993.pdf.
- ↑ Actually, knowing we would board a plane the next morning, we did not work terribly late on the 29th, breaking off sometime around 10 p.m. as I recall. I also specifically remember on the way home passing the scene of an accident I later learned claimed the life of one Jenny Lee Simpson. Her car had driven off the roadway, struck a large tree, and burst into flames. For many months as I drove to and from school, I passed the site of what is in my memory the first roadside memorial I had ever seen. I eventually dedicated my dissertation to the victim whom I never knew (mistakenly recording her last name as Thompson instead of Simpson) and to those who maintained the site.
- ↑ As described in Part I, a second batch of Y1.2Ba0.8CuO4 was synthesized in Huntsville on the 29th of January by heating for only a few hours in our anxiety to quickly reproduce our initial results. Note that the bottom of the page marked H54 in Meng’s Exhibit H Media:250MengExhibitH.pdf makes mention of what was for both Houston and Huntsville an unusually short sintering time (only 7 hours). Similarly, Chu’s desk calendar Media:440ChusCalendarFromRLM1224.pdf notes on 31 January, “Greatest Observation 20 min. is enough.” Thus, we are to believe by Meng’s declaration that Houston specifically chose to process Y1.2Ba0.8CuO4 samples using unusually short processing times even before making the first observation of superconductivity in any of the YBCO samples. With this, we can add shorter processing times to the list of remarkable Huntsville/Houston coincidences. Incidentally, the apparent benefits of the shorter processing times were likely a result of the fact that 1000 °C is ~75 °C above the optimal temperature for “solid state” processing of YBCO. During heating, some intermediate phases form with melting points around 890 °C (if my recollection is correct) that may tend to separate from the bulk. Thus, maximum temperatures are best limited to about 925 °C.
- ↑ See Media:810MengExhibitI.pdf.
- ↑ M. K. Wu, J. R. Ashburn, C. J. Torng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang, C. W. Chu. “Superconductivity at 93 K in a New Mixed-Phase Y-Ba-Cu-O Compound System at Ambient Pressure.” Phys. Rev. Lett. 58 (1987): 908-910 Media:310OriginalYBCOPaper.pdf.
- ↑ See endless discussions of this in Media:470MengAccountAshburnAnalysis.pdf.
- ↑ Barring the reality of some brilliant insights by Chu, perhaps like those documented in his previously examined past tense desk calendar (recall “yttrium has to work”).
- ↑ Bright colors (“rainbow colors” plus white) are characteristic of electrical insulators. Conductors (including superconductors) are more commonly black, gray, or any of the various shades commonly considered “metallic.”
- ↑ Media:480UAHSampleTestedInHouston19870130.jpg.
- ↑ R. Pool. “Superconductor Credits Bypass Alabama.” Science 5 (August 1988): 655-657. <http://science.sciencemag.org/content/241/4866/655>.
- ↑ James Riordon. “PRL Top Ten: #2, Superconductivity at 93K in a New Mixed-Phase Y-Ba-Cu-O Compound System.” <http://www.aps.org/publications/apsnews/200307/prl-2.cfm> accessed 5 June 2015. Incidentally, most regard Physical Review Letters as the world’s leading physics journal.
- ↑ By my recollection, two average sized labs separated by a suite of offices. Note that this was before the Texas Center for Superconductivity came into being.
- ↑ For whatever reason, on page 78 of her deposition Media:790MengDeposition93.pdf, Meng states that the compositions outlined on her notebook page marked 29 January were not even made until the period of 1-3 February. So much for the all-nighter.
- ↑ 2007 letter from Paul Chu to Richard Bannerot Media:820ChuLetterToBannerot.pdf.
- ↑ See pages marked 79 to 83 in the Meng deposition contained in Media:790MengDeposition93.pdf.
- ↑ C. W. Chu, L. Z. Deng, B. Lv. “Hole-Doped Cuprate High Temperature Superconductors.” Submitted on 16 Feb 2015. <http://arxiv.org/abs/1502.04686>. Accessed 14 March 2015. Accepted for publication in Physica C, Special Issue on Superconducting Materials.
- ↑ I suppose a third-person account makes it much easier to praise oneself. If there is lingering uncertainty about these being Chu’s words, the reader should compare Chu’s first-person variant of this same account that describes his “heavy personal involvement.” C. W. Chu. “Cuprates – Superconductors with a Tc up to 164 K.” Chapter 4.4, 100 Years of Superconductivity 1st Edition, ed. Horst Rogalla and Peter Kes, CRC Press/Taylor and Francis Group, Boca Raton, FL, 11 November 2011, page 245.
- ↑ Again, the reader is urged not to speculate that high-temperature superconductors have somehow enabled time travel.
- ↑ There is, of course, the previously-mentioned suspicious Y1.2Ba0.8CuO4 composition that appeared in Meng’s notebooks on the page with the date that first appeared redacted in Media:350MengDeclarationAndExhibitA1990.pdf and then later on unaltered as 15 January 1987 in Media:370MengExhibitF.jpg. Outside of the patent interference, the Houston team has been oddly disinclined to draw attention to this entry, as seems to be the case here.
- ↑ See Media:340MengMissingPagesPart1.pdf.
- ↑ See Media:330MengMissingPagesPart2.pdf.
- ↑ Incidentally, this failure in Houston occurred during the interval of time where, according to Chu’s various accounts, the Huntsville group had supposedly been tasked to do the yttrium work.
- ↑ C. W. Chu. “Superconductivity above 90 K.” Proc. Nati. Acad. Sci. USA 84 (July 1987): 4681-4682, paper presented at the symposium “Interfaces and Thin Films,” organized by J. Armstrong, D. E. Eastman, and G. M. Whitesides, March 23 and 24, 1987, at the National Academy of Sciences, Washington, D. C. <http://www.pnas.org/content/84/14/4681.short>.
- ↑ C. W. Chu. “The Brief History of RBCO.” The 20th Anniversary of the Discovery of RBCO. Denver, 5 March 2007 <http://www.w2agz.com/Presentations/2007/03-05%20APS%20March%20Denver/> Media:600Chu-070305HTS-APSDenver.pdf.
- ↑ Paul C. W. Chu. “From BCS through HTS to RTS.” BCS@50. Champaign Urbana, IL, 12 October 2007 <http://www.conferences.uiuc.edu/bcs50/PDF/Chu.pdf> Media:610Chu-101207BCSat50-Urbana.pdf.
- ↑ Paul C. W. Chu. “HTS 20 Years later: Achievements, Promises, Challenges plus the New Fe-Based HTS System.” 2008 American Physical Society, April Meeting, St. Louis MO, 14 April 2008 <http://apps3.aps.org/aps/meetings/april08/Q1.00003.pdf> Media:620PresentationQ100003.pdf.
- ↑ C. W. Chu. “Superconductivity above 90 K.” Proc. Nati. Acad. Sci. USA 84 (July 1987): 4681-4682, paper presented at the symposium “Interfaces and Thin Films,” organized by J. Armstrong, D. E. Eastman, and G. M. Whitesides, March 23 and 24, 1987, at the National Academy of Sciences, Washington, D. C. <http://www.pnas.org/content/84/14/4681.short>.
- ↑ R. J. Cava, “Oxide Superconductors,” J. Am. Ceram. Soc. 83.1 (2000): 5-28.
- ↑ Thus, I speculate that it may have been perceived that the discovery in Huntsville came as a result of us “getting out of our lane.”
- ↑ See <http://taiwanreview.nat.gov.tw/fp.asp?xItem=24060&CtNode=205> or <http://taiwantoday.tw/ct.asp?xItem=24060&CtNode=436> Media:830VolumeMatchingWuTaiwanToday2007.jpg.
- ↑ I have been repeatedly informed by several presumably knowledgeable friends that the term “work” used in this manner and context should never be mistaken for any mental or intellectual effort.
- ↑ Martin Burkey. “Wu Has Seen Good, Bad in Discovery.” The Huntsville Times (29 January 1988).
- ↑ L. Er-Rakho, C. Michel, J. Provost, B. Raveau. “The Oxides La3-xLnxBa3Cu(II)5-2yCu(III)1+2yO14+y.” Journal of Solid State Chemistry 37 (1981): 151-156 Media:070ErRakhoEtAlFromAshburn.jpg.
- ↑ After the early January conversation where the Houston team supposedly communicated with Wu the necessary and sufficient information by which the discovery was eventually achieved (see Appendix D for even more on this).
- ↑ For those who might suspect that the conversation recorded between Wu and myself was staged, please ask what would have been required to coerce Wu to confess to doctoring lab notebooks. If one questions whether or not the voice was actually that of Wu, I would recommend a comparison with the Wu video linked in Appendix B. Finally, if I were going to go to such great lengths to fake all of this evidence, surely I would have sense enough to create something better (a “smoking gun,” if you will) than a half gigabyte of mostly circumstantial evidence.
- ↑ See Media:150Y115Ba085CuO.jpg, Media:220Y12Ba08CuO.jpg, and the corresponding images in Melissa Ford Thornton, “Dawn of Discovery: UAH Discovery Stuns the World,” UAH Magazine 1.1 (Winter 1988): 4-7.
- ↑ With some valuable feedback from a group of undergraduate students by the names of Daniel Shultz, Tony Xidis, Jones Hamilton, and Raymond Cronise.
- ↑ <http://ieeemilestones.ethw.org/Milestone-Proposal:High_Temperature_Superconductivity>. Accessed 11 March 2015.
- ↑ See page 84 of C. W. Chu. “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc.” IEEE Transactions on Applied Superconductivity 7.2 (1997): 80-89. The details of the somewhat variable oxygen ratios were not established for several more weeks.
- ↑ Including the precise ratios of the metals necessary to calculate raw material weights toward synthesis of the materials. By Hazen’s account, the pure-phase superconductor was also known to be “perovskite-related” by the 27th, while more precise details were slowly cobbled together over the days and weeks to follow.
- ↑ Hazen dedicated an entire chapter (#5, 17 pages) to “The Green Phase.”
- ↑ Having planted this idea in the original YBCO paper, Chu continued to be reluctant to abandon the notion despite the overwhelming evidence against it (as almost every group working in the field over the months of December 1986 and January 1987, including Chu’s own Houston group, were producing relatively high purity single phase La2-xAexCuO4-type superconductors).
- ↑ R. M. Hazen, L. W. Finger, R. J. Angel, C. T. Prewitt, N. L. Ross, H. K. Mao, C. G. Hadichiacos, P. H. Hor, R. L. Meng, C. W. Chu. Phys. Rev. B 35 (1987): 7238.
- ↑ See page 84 of C. W. Chu. “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc.” IEEE Transactions on Applied Superconductivity 7.2 (1997): 80-89.
- ↑ It would seem bizarre to use the word “invented” here.
- ↑ Robert Pool. “Superconductor Patents: Four Groups Duke it Out.” Science 245.4921 (1 September 1989): 931-933.
- ↑ Again, see page 84 of C. W. Chu. “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc.” IEEE Transactions on Applied Superconductivity 7.2 (1997): 80-89.
- ↑ A. Khurana. “Superconductivity Seen Above the Boiling Point of Liquid Nitrogen.” Physics Today 40:4 (1987): 17-23 Media:510ObviousYttriumPhysicsToday.jpg.
- ↑ Voicemail left me by Paul Chu in 2001 Media:840ChuVoicemail20011214.mp3.
- ↑ As noted previously, several knowledgeable friends have informed me that the term “work” used in this manner and context has a very precise and limited meaning.
- ↑ C. W. Chu. “Superconductivity above 90 K.” Proc. Nati. Acad. Sci. USA 84 (July 1987): 4681-4682, paper presented at the symposium “Interfaces and Thin Films,” organized by J. Armstrong, D. E. Eastman, and G. M. Whitesides, March 23 and 24, 1987, at the National Academy of Sciences, Washington, D. C. <http://www.pnas.org/content/84/14/4681.short>.
- ↑ Janny Scott. “Resistance Movement : Breakthroughs in Electrical Superconductors Have Scientists Charged Up.” L. A. Times (5 April 1987). <http://articles.latimes.com/1987-04-05/local/me-416_1_superconductors> and page 2 <http://articles.latimes.com/1987-04-05/local/me-416_1_superconductors/2>, accessed 26 June 2015.
- ↑ R. J. Cava, “Oxide Superconductors,” J. Am. Ceram. Soc. 83.1 (2000): 5-28.
- ↑ Every toolbox should have at least one Occam’s Razor.
- ↑ G. Cowley, N. Abbott. “The Superman of Superconductivity.” Newsweek (19 December 1988): 63.
- ↑ Steven R. Reed. “As Xenophobes Posture, The Scientists Invent,” Page 2: “Superpowers Vie Over Super Scientists.” The Scientist 2.10 (30 May 1988): 1-2.
- ↑ I am guessing that Wu’s IP agreement with the university legally required him to do so.
- ↑ In Kelber’s retelling to me, he wrote boron instead of barium. I have assumed here that he intended the latter. It has been a few years.
- ↑ See page marked 582 in C. W. Chu, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Huang, and J. Bechtold. “Discovery and Physics of Superconductivity Above 90 K.” Novel Superconductivity: Proceedings of the International Symposium on Novel Mechanisms of Superconductivity. Ed. S. A. Wolf, V. Z. Kresin. New York: Plenum Press, 1987. 581-598. <http://link.springer.com/chapter/10.1007/978-1-4613-1937-5_68>.
- ↑ See Figure 2 on the page marked 20 in C. W. Chu. “Superconductivity Above 90 K and Beyond.” Proceedings of the 10th Anniversary HTS Workshop on Physics, Materials and Applications. Ed. B. Batlogg, C. W. Chu, W. K. Chu, D. U. Gubser, K. A. Müller. Singapore: World Scientific, 1996. 17 Media:560ChuSCAbove90K.pdf.
- ↑ C. W. Chu. “High Temperature Superconductivity.” History of Original Ideas and Basic Discoveries in Particle Physics. Ed. H. B. Newman, T. Ypsilantis. New York: Plenum, 1996. 793. <http://www.springer.com/us/book/9780306452178>.
- ↑ C. W. Chu. “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc.” IEEE Transactions on Applied Superconductivity 7.2 (1997): 80-89. <http://ieeexplore.ieee.org/xpl/login.jsp?tp=&arnumber=614424>.
- ↑ C. W. Chu. “The Brief History of RBCO.” The 20th Anniversary of the Discovery of RBCO. Denver, 5 March 2007 <http://www.w2agz.com/Presentations/2007/03-05%20APS%20March%20Denver/> Media:600Chu-070305HTS-APSDenver.pdf.
- ↑ Paul C. W. Chu. “From BCS through HTS to RTS.” BCS@50. Champaign Urbana, IL, 12 October 2007 <http://www.conferences.uiuc.edu/bcs50/PDF/Chu.pdf> Media:610Chu-101207BCSat50-Urbana.pdf.
- ↑ Paul C. W. Chu. “HTS 20 Years later: Achievements, Promises, Challenges plus the New Fe-Based HTS System.” 2008 American Physical Society, April Meeting, St. Louis MO, 14 April 2008 <http://apps3.aps.org/aps/meetings/april08/Q1.00003.pdf> Media:620PresentationQ100003.pdf.
- ↑ See page 2 of Meng’s 2006 “perjury” affidavit Media:760MengAffidavit2006.pdf.
- ↑ Personally, I need two to three months (after the New Year) to accomplish the transition.
- ↑ Perhaps it is an especially good thing that I never considered backdating any lab notebook entries. Mistakes can leave one looking rather foolish, and after some time I might even come to believe my own fantasies.
- ↑ Hor’s 2006 affidavit Media:770HorAffidavit2006.pdf.
- ↑ C. W. Chu. “Superconductivity above 90 K.” Proc. Nati. Acad. Sci. USA 84 (July 1987): 4681-4682, paper presented at the symposium “Interfaces and Thin Films,” organized by J. Armstrong, D. E. Eastman, and G. M. Whitesides, March 23 and 24, 1987, at the National Academy of Sciences, Washington, D. C. <http://www.pnas.org/content/84/14/4681.short>.
- ↑ Anthony Ramirez. “Superconductors Get Into Business.” Fortune (22 June 1987).
- ↑ T. H. Maugh. “New Material Further Increases Superconductor Temperature.” Los Angeles Times (23 June 1987).
- ↑ See Media:660RLM0572HoustonTests.pdf.
- ↑ C. W. Chu. “The Brief History of RBCO.” The 20th Anniversary of the Discovery of RBCO. Denver, 5 March 2007 <http://www.w2agz.com/Presentations/2007/03-05%20APS%20March%20Denver/> Media:600Chu-070305HTS-APSDenver.pdf.
- ↑ For the sake of the inset, the axis tick marks were painstakingly replicated on the interior of the plot area. This can be checked against the original.
- ↑ The fact that the Houston charts appeared to reach very near zero at 45 K after which the resistance increased with lower temperatures was the first red flag that prompted this more detailed examination.
- ↑ See page marked 802 in C. W. Chu. “High Temperature Superconductivity.” History of Original Ideas and Basic Discoveries in Particle Physics. Ed. H. B. Newman, T. Ypsilantis. New York: Plenum, 1996. 793. <http://www.springer.com/us/book/9780306452178>.
- ↑ S. Kar and R.G. Sharma. “Cryogenic Temperature Sensors.” Defence Science Journal, 57.3 (May 2007): 195-208.
- ↑ In an aps.org article, Chu is quoted, “At the time, thermometers in low temperature labs worldwide were seldom calibrated to above 25 K.” See James Riordon. “PRL Top Ten: #2, Superconductivity at 93K in a New Mixed-Phase Y-Ba-Cu-O Compound System.” <http://www.aps.org/publications/apsnews/200307/prl-2.cfm> accessed 5 June 2015.
- ↑ Neglecting the possibility that the device might have also been capable of Hall effect measurements.
- ↑ See page marked 802 in C. W. Chu. “High Temperature Superconductivity.” History of Original Ideas and Basic Discoveries in Particle Physics. Ed. H. B. Newman, T. Ypsilantis. New York: Plenum, 1996. 793. <http://www.springer.com/us/book/9780306452178>.
- ↑ My extrapolation predicts R(4.2 K) of 305.0 Ω, consistent with the very highest resistance reading (~302 Ω in the first curve), presumably at or near the LHe temperature.
- ↑ One full span was very commonly but not universally the convention within the larger set of Houston data in my possession.
- ↑ G. Bednorz and K. A. Müller, “Possible High Tc Superconductivity in the Ba-La-Cu-O System,” Z. Phys. B, Condensed Matter 64 (1986): 189-193.
- ↑ See page marked 802 in C. W. Chu. “High Temperature Superconductivity.” History of Original Ideas and Basic Discoveries in Particle Physics. Ed. H. B. Newman, T. Ypsilantis. New York: Plenum, 1996. 793. <http://www.springer.com/us/book/9780306452178>.
- ↑ His website can be seen at <http://www.w2agz.com>. The reader might also note his disclaimer: <http://www.w2agz.com/About%20This%20Site.htm>.
- ↑ Paul Chu. Presentation at the Spring American Physical Society Meeting, New York: 18 March 1987 880V19870318APSChu.mp4.
- ↑ C. W. Chu. “Superconductivity above 90 K.” Proc. Nati. Acad. Sci. USA 84 (July 1987): 4681-4682, paper presented at the symposium “Interfaces and Thin Films,” organized by J. Armstrong, D. E. Eastman, and G. M. Whitesides, March 23 and 24, 1987, at the National Academy of Sciences, Washington, D. C. <http://www.pnas.org/content/84/14/4681.short>.
- ↑ Paul Chu. Presentation at the Spring Materials Research Society Meeting, Anaheim: 21-25 April 1987 890V1987SpringMRSAnaheimChu.mp4.
- ↑ Maw-Kuen Wu. Presentation at the Spring Materials Research Society Meeting, Anaheim: 21-25 April 1987 900V1987SpringMRSAnaheimWu.mp4.
- ↑ Paul Chu. Presentation at the “Novel Superconductivity” Berkeley Workshop, June 1987 910V1987JuneBerkeleyWorkshopChu.mp4.
- ↑ See C. W. Chu, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Huang, J. Bechtold. “Discovery and Physics of Superconductivity Above 90 K.” Novel Superconductivity: Proceedings of the International Symposium on Novel Mechanisms of Superconductivity. Ed. S. A. Wolf, V. Z. Kresin. New York: Plenum Press, 1987. 581-598. <http://link.springer.com/chapter/10.1007/978-1-4613-1937-5_68>. In a personal email from Vladamir Kresin, I learned that a hasty “reorganization” of the meeting (originally planned before the flurry that began in early December ‘86) allowed for papers to be submitted after the presentations. Incidentally, the proceedings were very nicely hardbound.
- ↑ See Hor email Media:720HorEmail.jpg.
- ↑ Consistent with the presence of ferromagnetic contamination, but this is speculation, albeit no less valid and far more plausible than claims of evidence for superconductivity in this data.
- ↑ C. Y. Huang, L. J. Dries, P. H. Hor, R. L. Meng, C. W. Chu, and R. B. Frankel. “Observation of possible superconductivity at 230 K.” Nature 328 (30 July 1987): 403-404. <http://www.nature.com/nature/journal/v328/n6129/abs/328403a0.html> and for download from a co-author’s site at <http://works.bepress.com/rfrankel/142/>.
- ↑ See page marked 592 of C. W. Chu, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Huang, J. Bechtold. “Discovery and Physics of Superconductivity Above 90 K.” Novel Superconductivity: Proceedings of the International Symposium on Novel Mechanisms of Superconductivity. Ed. S. A. Wolf, V. Z. Kresin. New York: Plenum Press, 1987. 581-598. <http://link.springer.com/chapter/10.1007/978-1-4613-1937-5_68>.
- ↑ Thermocouple reference junctions are frequent culprits.
- ↑ See the page marked RLM0165 in the 2007 letter from Paul Chu to Richard Bannerot Media:820ChuLetterToBannerot.pdf.
- ↑ Unsubmitted 1990 Chu declaration Media:920ChuDeclaration1990.pdf.
- ↑ Recalling my description in Part I of Wu’s spring ‘87 colloquium, I have never understood what mental processes require some to highlight the imagined incompetence of others. Surely a person of truly superior intelligence, especially one in a position of authority, would surround himself with similarly competent colleagues.
- ↑ By Chu’s account, not even the muses are worthy of a citation.
- ↑ R. J. Cava, “Oxide Superconductors,” J. Am. Ceram. Soc. 83.1 (2000): 5-28.
- ↑ Final signed but unfiled 1990 Chu declaration Media:925ChuDeclarationFinal1990.pdf.
- ↑ Within weeks of initiating my writing of this document, I began to have severe reflux symptoms. Eight months in I have discovered that the severity of my symptoms is actually attributable to what had progressed to 24/7 premature atrial contractions (a heart arrhythmia) triggered by a severely irritated esophagus. The reader might discern that the effects of such an ordeal upon one’s health is the primary reason I would rather die than take this matter up in a court of law, although I fear that some may be tempted to force such an outcome in order to drag me yet again through hell.
- ↑ Understatement.
- ↑ Media:960HorVChuFindingsOfFactAndConclusionsOfLaw.pdf.
- ↑ “Download Official Documents – Dr. Pei-Hreng (sic.) Hor v. Dr. Ching-Wu ‘Paul’ Chu.” RFC Express: U. S. Federal District Court Recently Filed Cases. < http://www.rfcexpress.com/lawsuits/patent-lawsuits/texas-southern-district-court/42499/dr-pei-hreng-hor-v-dr-ching-wu-paul-chu/official-court-documents/>, accessed 26 June 2015. In the event the link changes, the full case name and number are “Pei-Herng Hor, Plaintiff, v. Ching-Wu ‘Paul’ Chu Defendant,” Civil Action No. 4:08-cv-3584.” The documents can also be downloaded from the government web site <https://www.pacer.gov/>.
- ↑ The reader will have to review these topics in the main narrative for the appropriate citations. My energy is waning.
- ↑ Media:960HorVChuFindingsOfFactAndConclusionsOfLaw.pdf.
- ↑ A bit bizarre given that the actual idea was far from any Nobel quality act of genius.
- ↑ Recall from Appendix C that, as best I can determine, this declaration was never actually submitted. It is unclear to me whether or not the Federal District Court was aware of this fact.
- ↑ C. W. Chu, L. Z. Deng, B. Lv. “Hole-Doped Cuprate High Temperature Superconductors.” Submitted on 16 Feb 2015. <http://arxiv.org/abs/1502.04686>. Accessed 14 March 2015. Accepted for publication in Physica C, Special Issue on Superconducting Materials.
- ↑ December 26th entry in Chu’s desk calendar Media:440ChusCalendarFromRLM1224.pdf.
- ↑ “Corrected Brief of Appellant Pei-Herng Hor” filed 18 April 2012 Media:970CorrectedBriefOfHor20120418.pdf.
- ↑ See Media:740MengExhibitG.jpg.
- ↑ See Media:740MengExhibitG.jpg.
- ↑ See Media:330MengMissingPagesPart2.pdf.
- ↑ See page 808 of C. W. Chu. “High-Temperature Superconducting Materials: A Decade of Impressive Advancement of Tc.” IEEE Transactions on Applied Superconductivity 7.2 (1997): 80-89. <http://ieeexplore.ieee.org/xpl/login.jsp?tp=&arnumber=614424>.
- ↑ If my recollection is correct, I believe that there was one joint paper by Houston in the weeks to follow that listed Wu, but the relationship between the two schools decayed rapidly.
- ↑ Ruling Meng’s Exhibit H from the patent interference proceedings, excerpts from her lab notebook beginning late January 1987 Media:250MengExhibitH.pdf.
- ↑ Gina Kolata. “Yb or Not Yb? That is the Question.” Science, 236 (8 May 1987):663-4. <http://science.sciencemag.org/content/236/4802/663>.
- ↑ See Media:260MengExhibitHFirstPage.jpg.
- ↑ This point was also learned from me by Hor during exchanges beginning around 2006.
- ↑ Yes, given that the ratios and processing conditions are not discrete, it is technically worse than a combinatorial problem. I am assuming here ratios at some reasonable discrete steps and over finite ranges.
- ↑ See Media:260MengExhibitHFirstPage.jpg.
- ↑ See Media:990Exhibit1MengDeposition20100512.pdf.
- ↑ See Media:1000BriefOfChu20111221.pdf.
- ↑ See Media:1010ChusReply20140530.pdf.
- ↑ D. H. Bowen. in High Pressure Physics and Chemistry I. ed. R. S. Bradley, Academic Press, Inc., New York (1963): 355-373.
- ↑ H. Kamimura, H. Ushio, S. Matsuno, T. Hamada. Theory of Copper Oxide Superconductors. Heidelberg: Springer, 2005.
- ↑ Hideki Ushio. “On the Interplay of Jahn-Teller Physics and Mott Physics in the Mechanism of High Tc Superconductivity.” Presented at the XXth International Symposium on the Jahn-Teller Effect. Fribourg, Switzerland (17-20 August 2010). <http://www.unifr.ch/jt2010/ushio.pdf>.
- ↑ His fear of blood and needles meant that medicine was an almost certain path to failure anyway.
- ↑ Many thanks will forever be due Jim Smith and James “Danny” Doss (1939-2012) for treating me like royalty during my interview. The opportunity to enjoy dinner with Jim and his wife at their home along with their other guest that week, Edward Teller, was a truly unforgettable experience.